HAEM4:Acute Myeloid Leukemia (AML) and Related Precursor Neoplasms
Primary Author(s)*
Daniel Butler, MD
Daynna J. Wolff, PhD
Graphical Data Links
AML Table - A comprehensive list of CNAs and CN-LOH detectable by CMA testing with strong diagnostic, prognostic and treatment implications in AML. Table derived from Xu et al., 2018 [PMID 30344013] with permission from Cancer Genetics. See AML Table: Recurrent Genomic Alterations Detected by Chromosomal Microarray.
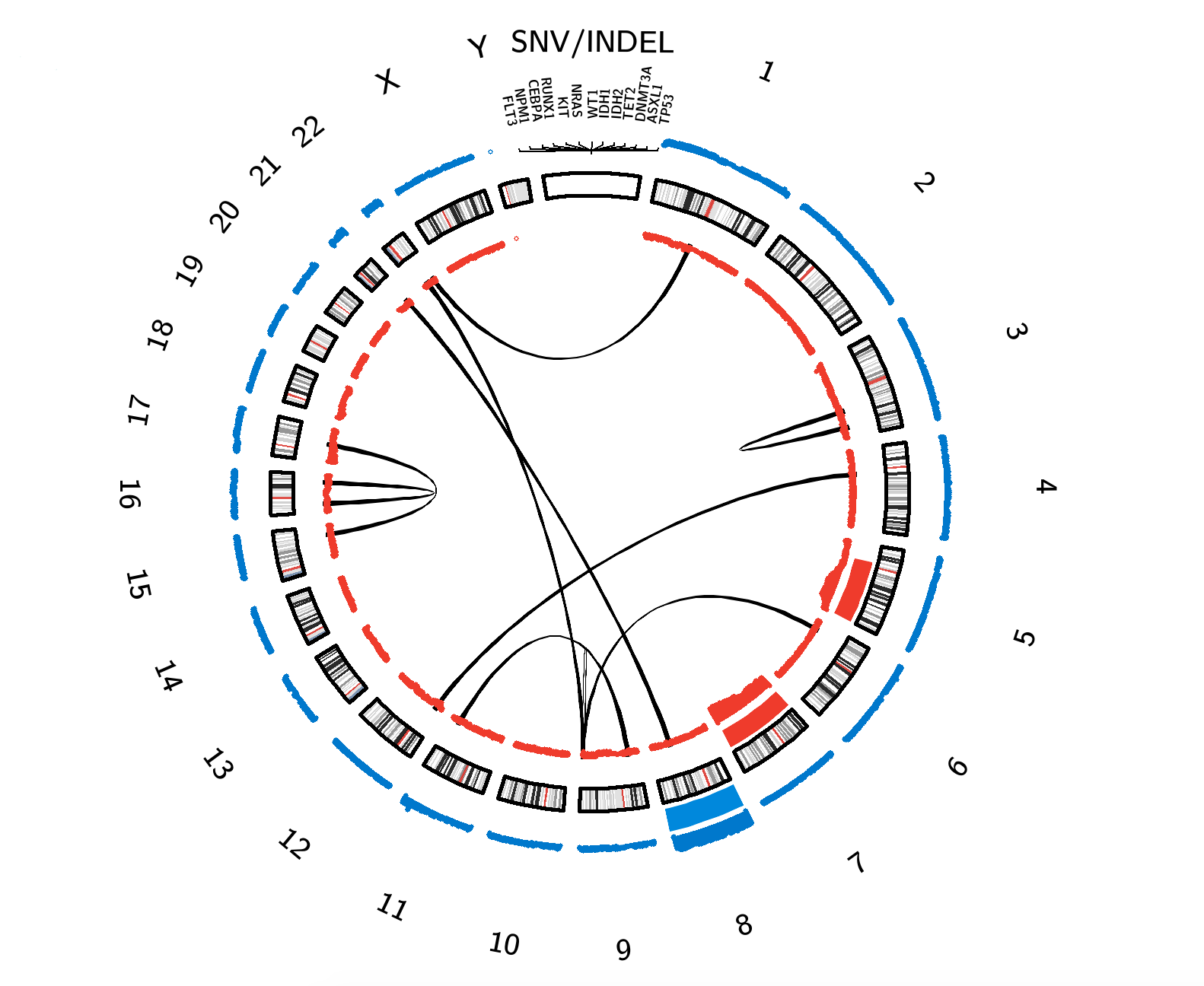
AML Circos Plot. Click on interactive content to be linked to related pages. Lines = Structural Rearrangements (between connected chromosomes); Gene Names = Gene-Specific Alterations; Red Bars = Copy Number Losses; Blue Bars = Copy Number Gains. The thickness of the red and blue bars correlates with the corresponding copy number change frequency.
TCGA Data on AML. Red Bars = Copy Number Losses; Blue Bars = Copy Number Gains. The height of the red and blue bars correlates with the corresponding copy number change frequency (additional information obtained by hovering over area of interest).
General Disease Overview Description of Cancer Category
The major classification group of AML is "AML and Related Precursor Neoplasms", as described in the revised 4th edition of the WHO[1], which includes the categories: AML with Recurrent Genetic Abnormalities, AML with Myelodysplasia-Related Changes, Therapy-Related Myeloid Neoplasms, AML NOS, Myeloid Sarcoma and Myeloid Proliferations Associated with Down Syndrome. Links to these categories as well as links to subcategories are listed below in the "WHO Classification Pages" section. In addition, significant genetic topics related to AML not currently considered a WHO-defined entity are also listed below in the "Other Related Pages" section. A summary of the changes to the revised 4th edition from the 2008 WHO edition has also recently been described[2].
Acute myeloid leukemia (AML) represents a clonal proliferation of abnormal precursor cells of myeloid lineage. These leukemic cells lack maturation and do not function like their differentiated counterparts such as neutrophils, but rather rapidly proliferate in the marrow space and replace physiologic hematopoiesis. As a consequence, patients typically present with symptoms of pancytopenia, including fatigue, infection, and bleeding. According to the American Cancer Society, roughly 60,300 new cases of leukemia will be diagnosed in 2018, 32% of which will be due to AML. AML is predominantly seen in adults with a median age of 65 years and is more common in white males[3] and is is less common in pediatric populations, accounting for approximately 18% of childhood leukemias[4]. The incidence of pediatric AML peaks in the first year of life, declines in years 5-9, and then increases again in years 10-15[5]. Trisomy 21 has been reported as the most common genetic factor for pediatric AML, which has been associated with a 500-fold risk in the development of the AML, particularly the acute megakaryocytic leukemia subtype[5]. Several inherited genetic disorders also have an increased risk for AML including Fanconi anemia, familial monosomy 7, and in utero radiation[5]. In adults, risk factors include environmental exposures (e.g., radiation, tobacco use, chemotherapy) and preceding myelodysplastic syndromes or myeloproliferative neoplasms[6][7].
AML is characterized by molecular and cytogenetic heterogeneity that informs prognosis and clinical management. Translocations and inversions are common and typically result in structural rearrangements that create gene fusions that prohibit maturation or promote unregulated proliferation. For example, acute promyelocytic leukemia (APL) is characterized by a t(15;17)(q24.1;q21.1) that fuses the promyelocytic leukemia (PML) gene at 15q24.1 to the retinoic acid alpha-receptor (RARA) gene at 17q21.1, resulting in an accumulation of promyelocytes that cannot undergo differentiation into granulocytes. All-trans-retinoic acid (ATRA) and arsenic are two therapies useful in disrupting PML-RARA gene fusions and promoting promyelocytic differentiation, thereby preventing life-threatening complications such as fulminant disseminated intravascular coagulation (DIC).
In addition to cytogenetic anomalies, mutations are likewise prevalent in AML[8][9]. These mutations offer prognostic value regarding disease progression and recurrence and are evaluated alongside other prognostic factors, including age, performance status, chemoradiation history, and prior myelodysplastic disease[1].
WHO Classification Pages (Includes Links to Content)
- AML with t(8;21)(q22;q22.1); RUNX1-RUNX1T1 - AML with with inv(16)(p13.1q22) or t(16;16)(p13.1;q22); CBFB-MYH11 - HAEM5:Acute promyelocytic leukaemia with PML::RARA fusion - AML with t(9;11)(p21.3;q23.3); KMT2A-MLLT3 - AML with t(6;9)(p23;q34.1); DEK-NUP214 - AML with inv(3)(q21.3q26.2) or t(3;3)(q21.3;q26.2);GATA2, MECOM - AML Megakaryoblastic with t(1;22)(p13.3;q13.1);RBM15-MKL1 - AML with BCR-ABL1 - AML with Mutated NPM1 - AML with Biallelic Mutations of CEBPA - AML with Mutated RUNX1
- AML with Minimal Differentiation - AML without Maturation - AML with Maturation - HAEM5:Acute myelomonocytic leukaemia - HAEM5:Acute monocytic leukaemia - HAEM5:Acute erythroid leukaemia - HAEM5:Acute megakaryoblastic leukaemia - HAEM5:Acute basophilic leukaemia - HAEM4:Acute Panmyelosis with Myelofibrosis
- HAEM5:Myeloid proliferations associated with Down syndrome - HAEM5:Myeloid proliferations associated with Down syndrome
- HAEM5:Acute undifferentiated leukaemia - HAEM5:Mixed-phenotype acute leukaemia with BCR::ABL1 fusion - HAEM5:Mixed-phenotype acute leukaemia with KMT2A rearrangement - HAEM5:Mixed-phenotype acute leukaemia, B/myeloid - HAEM5:Mixed-phenotype acute leukaemia, T/myeloid - HAEM5:Mixed-phenotype acute leukaemia, rare types - HAEM4:Acute Leukemias of Ambiguous Lineage, Not Otherwise Specified (NOS)
- HAEM4:Acute Myeloid Leukaemia with Germline CEBPA Mutation - HAEM4:Myeloid Neoplasms with Germline DDX41 Mutation - HAEM4:Myeloid Neoplasms with Germline RUNX1 Mutation - HAEM4:Myeloid Neoplasms with Germline ANKRD26 Mutation - HAEM4:Myeloid Neoplasms with Germline ETV6 Mutation - HAEM4:Myeloid Neoplasms with Germline GATA2 Mutation
Other Related Pages (Includes Links to Content)
Additional Information
Put your text here
References
- ↑ 1.0 1.1 Acute myeloid leukaemia with related precursor neoplasms, in World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues, Revised 4th edition. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Arber DA, Hasserjian RP, Le Beau MM, Orazi A, and Siebert R, Editors. IARC Press: Lyon, France, p122-187, 2017.
- ↑ Vardiman, James W.; et al. (2009-07-30). "The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes". Blood. 114 (5): 937–951. doi:10.1182/blood-2009-03-209262. ISSN 1528-0020. PMID 19357394.
- ↑ American Cancer Society. Cancer Facts & Figures 2018. Atlanta, Ga: American Cancer Society; 2018.
- ↑ Linabery, Amy M.; et al. (2008-01-15). "Trends in childhood cancer incidence in the U.S. (1992-2004)". Cancer. 112 (2): 416–432. doi:10.1002/cncr.23169. ISSN 0008-543X. PMID 18074355.
- ↑ 5.0 5.1 5.2 Puumala, Susan E.; et al. (2013-05). "Epidemiology of childhood acute myeloid leukemia". Pediatric Blood & Cancer. 60 (5): 728–733. doi:10.1002/pbc.24464. ISSN 1545-5017. PMC 3664189. PMID 23303597. Check date values in:
|date=(help) - ↑ https://www.uptodate.com/contents/pathogenesis-of-acute-myeloid-leukemia
- ↑ "Risk Factors for Acute Myeloid Leukemia (AML)".
- ↑ DiNardo, Courtney D.; et al. (2016-12-02). "Mutations in AML: prognostic and therapeutic implications". Hematology. American Society of Hematology. Education Program. 2016 (1): 348–355. doi:10.1182/asheducation-2016.1.348. ISSN 1520-4383. PMC 6142505. PMID 27913501.
- ↑ Yang, Fei; et al. (2020-02). "Clinical Utility of Next-Generation Sequencing in Acute Myeloid Leukemia". Molecular Diagnosis & Therapy. 24 (1): 1–13. doi:10.1007/s40291-019-00443-9. ISSN 1179-2000. PMID 31848884. Check date values in:
|date=(help)
Notes
*Primary authors will typically be those that initially create and complete the content of a page. If a subsequent user modifies the content and feels the effort put forth is of high enough significance to warrant listing in the authorship section, please contact the CCGA coordinators (contact information provided on the homepage). Additional global feedback or concerns are also welcome.
*The hierarchical tumour classification structure displayed on this page is reproduced from the WHO Classification of Tumours with permission from the copyright holder, ©International Agency for Research on Cancer.