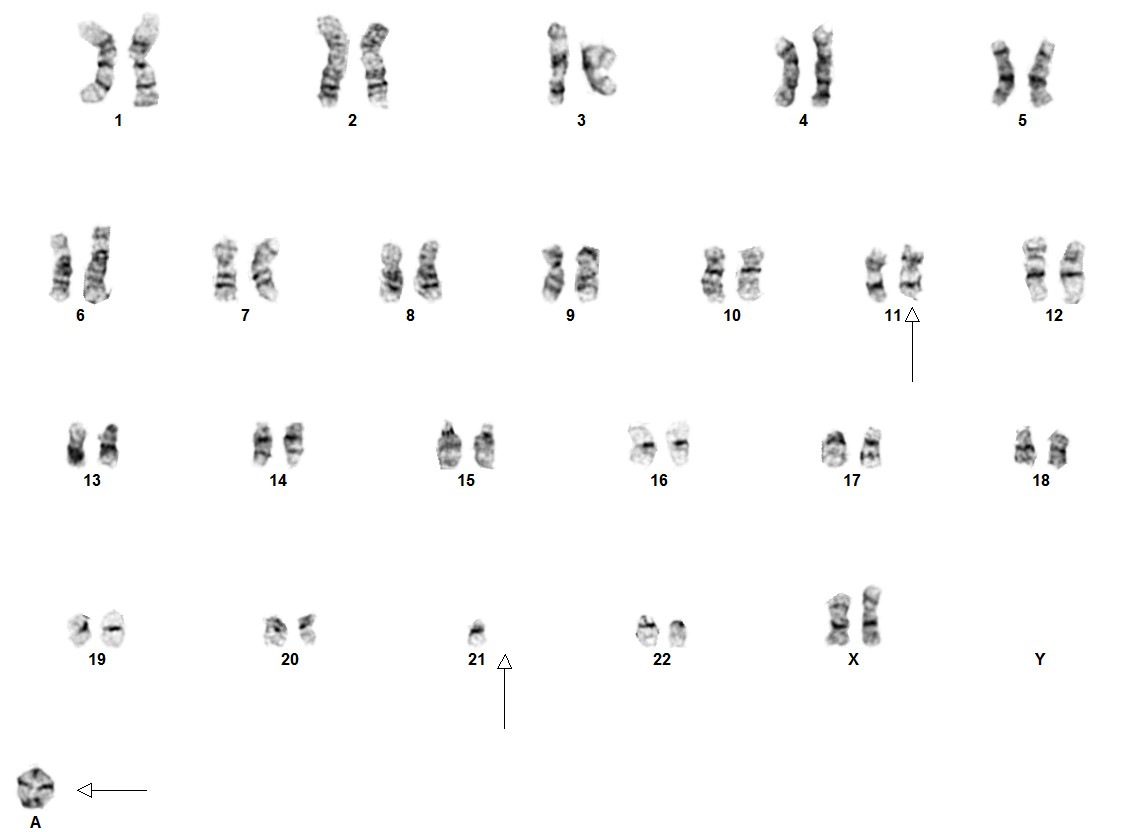
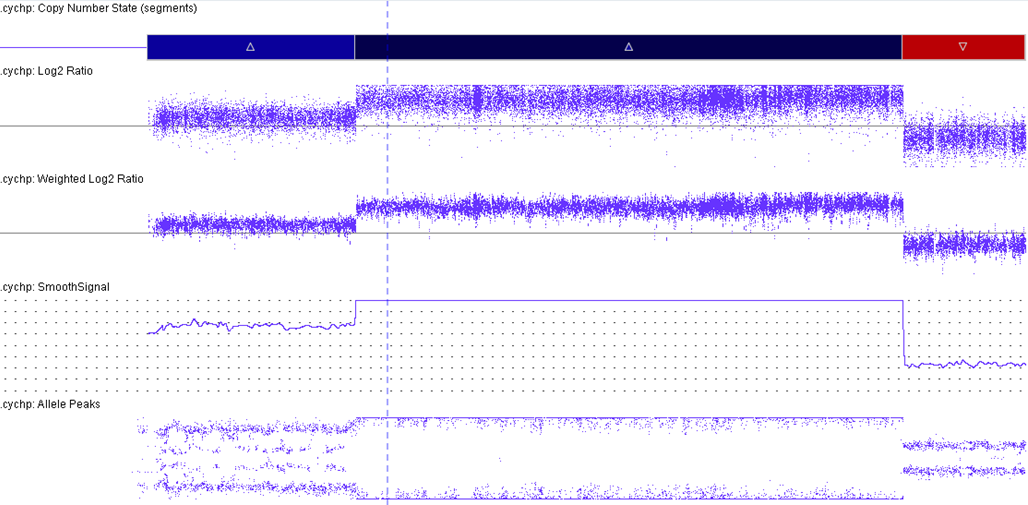
B-lymphoblastic leukaemia/lymphoma with iAMP21
Haematolymphoid Tumours (WHO Classification, 5th ed.)
Primary Author(s)*
Holli M. Drendel, PhD, FACMGG, Carolinas Pathology Group, Charlotte
WHO Classification of Disease
| Structure | Disease |
|---|---|
| Book | Haematolymphoid Tumours (5th ed.) |
| Category | B-cell lymphoid proliferations and lymphomas |
| Family | Precursor B-cell neoplasms |
| Type | B-lymphoblastic leukaemias/lymphomas |
| Subtype(s) | B-lymphoblastic leukaemia/lymphoma with iAMP21 |
WHO Essential and Desirable Genetic Diagnostic Criteria
(Instructions: The table will have the diagnostic criteria from the WHO book autocompleted; remove any non-genetics related criteria. If applicable, add text about other classification systems that define this entity and specify how the genetics-related criteria differ.)
| WHO Essential Criteria (Genetics)* | |
| WHO Desirable Criteria (Genetics)* | |
| Other Classification |
*Note: These are only the genetic/genomic criteria. Additional diagnostic criteria can be found in the WHO Classification of Tumours.
Related Terminology
(Instructions: The table will have the related terminology from the WHO autocompleted.)
| Acceptable | |
| Not Recommended |
Gene Rearrangements
Put your text here and fill in the table (Instructions: Details on clinical significance such as prognosis and other important information can be provided in the notes section. Please include references throughout the table. Do not delete the table.)
| Driver Gene | Fusion(s) and Common Partner Genes | Molecular Pathogenesis | Typical Chromosomal Alteration(s) | Prevalence -Common >20%, Recurrent 5-20% or Rare <5% (Disease) | Diagnostic, Prognostic, and Therapeutic Significance - D, P, T | Established Clinical Significance Per Guidelines - Yes or No (Source) | Clinical Relevance Details/Other Notes |
|---|---|---|---|---|---|---|---|
| EXAMPLE: ABL1 | EXAMPLE: BCR::ABL1 | EXAMPLE: The pathogenic derivative is the der(22) resulting in fusion of 5’ BCR and 3’ABL1. | EXAMPLE: t(9;22)(q34;q11.2) | EXAMPLE: Common (CML) | EXAMPLE: D, P, T | EXAMPLE: Yes (WHO, NCCN) | EXAMPLE:
The t(9;22) is diagnostic of CML in the appropriate morphology and clinical context (add reference). This fusion is responsive to targeted therapy such as Imatinib (Gleevec) (add reference). BCR::ABL1 is generally favorable in CML (add reference). |
| EXAMPLE: CIC | EXAMPLE: CIC::DUX4 | EXAMPLE: Typically, the last exon of CIC is fused to DUX4. The fusion breakpoint in CIC is usually intra-exonic and removes an inhibitory sequence, upregulating PEA3 genes downstream of CIC including ETV1, ETV4, and ETV5. | EXAMPLE: t(4;19)(q25;q13) | EXAMPLE: Common (CIC-rearranged sarcoma) | EXAMPLE: D | EXAMPLE:
DUX4 has many homologous genes; an alternate translocation in a minority of cases is t(10;19), but this is usually indistinguishable from t(4;19) by short-read sequencing (add references). | |
| EXAMPLE: ALK | EXAMPLE: ELM4::ALK
|
EXAMPLE: Fusions result in constitutive activation of the ALK tyrosine kinase. The most common ALK fusion is EML4::ALK, with breakpoints in intron 19 of ALK. At the transcript level, a variable (5’) partner gene is fused to 3’ ALK at exon 20. Rarely, ALK fusions contain exon 19 due to breakpoints in intron 18. | EXAMPLE: N/A | EXAMPLE: Rare (Lung adenocarcinoma) | EXAMPLE: T | EXAMPLE:
Both balanced and unbalanced forms are observed by FISH (add references). | |
| EXAMPLE: ABL1 | EXAMPLE: N/A | EXAMPLE: Intragenic deletion of exons 2–7 in EGFR removes the ligand-binding domain, resulting in a constitutively active tyrosine kinase with downstream activation of multiple oncogenic pathways. | EXAMPLE: N/A | EXAMPLE: Recurrent (IDH-wildtype Glioblastoma) | EXAMPLE: D, P, T | ||
Some rearrangements have been seen as secondary abnormalities.
| Chromosomal Rearrangement | Genes in Fusion (5’ or 3’ Segments) | Pathogenic Derivative | Prevalence | Diagnostic Significance (Yes, No or Unknown) | Prognostic Significance (Yes, No or Unknown) | Therapeutic Significance (Yes, No or Unknown) | Notes |
|---|---|---|---|---|---|---|---|
| del(X)(p22.33p22.33)/del(Y)(p11.32p11.32) | P2RY8-CRLF2 | der(X)/der(Y) | Because of the unique nature of the iAMP21 abnormality, cases that present with additional genomic lesions that may suggest another category, such as a CRLF2 rearrangement, should still be classified as B-ALL with iAMP21. | ||||
| t(12;21)(p13.2;q22.1) | ETV6-RUNX1 | der(21) | |||||
| t(9;22)(q34;q11.2) | BCR-ABL1 | der(22) |
Individual Region Genomic Gain/Loss/LOH
Put your text here and fill in the table (Instructions: Includes aberrations not involving gene rearrangements. Details on clinical significance such as prognosis and other important information can be provided in the notes section. Can refer to CGC workgroup tables as linked on the homepage if applicable. Please include references throughout the table. Do not delete the table.)
| Chr # | Gain, Loss, Amp, LOH | Minimal Region Cytoband and/or Genomic Coordinates [Genome Build; Size] | Relevant Gene(s) | Diagnostic, Prognostic, and Therapeutic Significance - D, P, T | Established Clinical Significance Per Guidelines - Yes or No (Source) | Clinical Relevance Details/Other Notes |
|---|---|---|---|---|---|---|
| EXAMPLE:
7 |
EXAMPLE: Loss | EXAMPLE:
chr7 |
EXAMPLE:
Unknown |
EXAMPLE: D, P | EXAMPLE: No | EXAMPLE:
Presence of monosomy 7 (or 7q deletion) is sufficient for a diagnosis of AML with MDS-related changes when there is ≥20% blasts and no prior therapy (add reference). Monosomy 7/7q deletion is associated with a poor prognosis in AML (add references). |
| EXAMPLE:
8 |
EXAMPLE: Gain | EXAMPLE:
chr8 |
EXAMPLE:
Unknown |
EXAMPLE: D, P | EXAMPLE:
Common recurrent secondary finding for t(8;21) (add references). | |
| EXAMPLE:
17 |
EXAMPLE: Amp | EXAMPLE:
17q12; chr17:39,700,064-39,728,658 [hg38; 28.6 kb] |
EXAMPLE:
ERBB2 |
EXAMPLE: D, P, T | EXAMPLE:
Amplification of ERBB2 is associated with HER2 overexpression in HER2 positive breast cancer (add references). Add criteria for how amplification is defined. | |
Cytogenetic morphology of the abnormal chromosome 21 can vary markedly between patients.[1]
In ~80% of iAMP21 B-ALL cases, recurrent secondary abnormalities, both chromosomal and molecular, have been documented. Deletions involving particular genes such as; IKZF1, CDKN2A/B, PAX5, SH2B3, ETV6 and RB1 have also been observed.
| Chr # | Gain / Loss / Amp / LOH | Minimal Region Genomic Coordinates [Genome Build] | Minimal Region Cytoband | Diagnostic Significance (Yes, No or Unknown) | Prognostic Significance (Yes, No or Unknown) | Therapeutic Significance (Yes, No or Unknown) | Notes |
|---|---|---|---|---|---|---|---|
| X | Gain | EXAMPLE:
chr7:1- 159,335,973 [hg38] |
EXAMPLE:
chr7 |
Yes | Yes | No | EXAMPLE:
Presence of monosomy 7 (or 7q deletion) is sufficient for a diagnosis of AML with MDS-related changes when there is ≥20% blasts and no prior therapy (add reference). Monosomy 7/7q deletion is associated with a poor prognosis in AML (add reference). |
| 10 | Gain | EXAMPLE:
chr8:1-145,138,636 [hg38] |
EXAMPLE:
chr8 |
No | No | No | EXAMPLE:
Common recurrent secondary finding for t(8;21) (add reference). |
| 14 | Gain | ||||||
| 7/7q | Loss | ||||||
| 11q | Loss |
Characteristic Chromosomal or Other Global Mutational Patterns
Put your text here and fill in the table (Instructions: Included in this category are alterations such as hyperdiploid; gain of odd number chromosomes including typically chromosome 1, 3, 5, 7, 11, and 17; co-deletion of 1p and 19q; complex karyotypes without characteristic genetic findings; chromothripsis; microsatellite instability; homologous recombination deficiency; mutational signature pattern; etc. Details on clinical significance such as prognosis and other important information can be provided in the notes section. Please include references throughout the table. Do not delete the table.)
| Chromosomal Pattern | Molecular Pathogenesis | Prevalence -
Common >20%, Recurrent 5-20% or Rare <5% (Disease) |
Diagnostic, Prognostic, and Therapeutic Significance - D, P, T | Established Clinical Significance Per Guidelines - Yes or No (Source) | Clinical Relevance Details/Other Notes |
|---|---|---|---|---|---|
| EXAMPLE:
Co-deletion of 1p and 18q |
EXAMPLE: See chromosomal rearrangements table as this pattern is due to an unbalanced derivative translocation associated with oligodendroglioma (add reference). | EXAMPLE: Common (Oligodendroglioma) | EXAMPLE: D, P | ||
| EXAMPLE:
Microsatellite instability - hypermutated |
EXAMPLE: Common (Endometrial carcinoma) | EXAMPLE: P, T | |||
iAMP21 cases have a characteristic pattern that is both complex and variable. This pattern comprises multiple regions of gain, amplification and deletion. Interestingly, RUNX1 amplification is not always intrachromosomal.[2][3] The formation of iAMP21 is considered to be due to breakage-fusion-bridge cycles followed by chromothripsis and other complex structural rearrangements of chromosome 21. Studies, molecular and cytogenetic, have elucidated a common 5.1 Mb region that includes the RUNX1 gene. However, even though RUNX1 is included in the amplified region, there has not yet been any conclusive evidence that RUNX1 is critical in the pathogenesis of disease given that it is not overexpressed in some individuals with this abnormality.[4][5][6]
| Chromosomal Pattern | Diagnostic Significance (Yes, No or Unknown) | Prognostic Significance (Yes, No or Unknown) | Therapeutic Significance (Yes, No or Unknown) | Notes |
|---|---|---|---|---|
| Amplification of RUNX1 | See CMA image displaying the amplification of RUNX1. This is part of the critical region consistently amplified: chr21:32.8-37.9 Mb (hg19). | |||
| Terminal deletion of 21q |
Gene Mutations (SNV/INDEL)
Put your text here and fill in the table (Instructions: This table is not meant to be an exhaustive list; please include only genes/alterations that are recurrent or common as well either disease defining and/or clinically significant. If a gene has multiple mechanisms depending on the type or site of the alteration, add multiple entries in the table. For clinical significance, denote associations with FDA-approved therapy (not an extensive list of applicable drugs) and NCCN or other national guidelines if applicable; Can also refer to CGC workgroup tables as linked on the homepage if applicable as well as any high impact papers or reviews of gene mutations in this entity. Details on clinical significance such as prognosis and other important information such as concomitant and mutually exclusive mutations can be provided in the notes section. Please include references throughout the table. Do not delete the table.)
| Gene | Genetic Alteration | Tumor Suppressor Gene, Oncogene, Other | Prevalence -
Common >20%, Recurrent 5-20% or Rare <5% (Disease) |
Diagnostic, Prognostic, and Therapeutic Significance - D, P, T | Established Clinical Significance Per Guidelines - Yes or No (Source) | Clinical Relevance Details/Other Notes |
|---|---|---|---|---|---|---|
| EXAMPLE:EGFR
|
EXAMPLE: Exon 18-21 activating mutations | EXAMPLE: Oncogene | EXAMPLE: Common (lung cancer) | EXAMPLE: T | EXAMPLE: Yes (NCCN) | EXAMPLE: Exons 18, 19, and 21 mutations are targetable for therapy. Exon 20 T790M variants cause resistance to first generation TKI therapy and are targetable by second and third generation TKIs (add references). |
| EXAMPLE: TP53; Variable LOF mutations
|
EXAMPLE: Variable LOF mutations | EXAMPLE: Tumor Supressor Gene | EXAMPLE: Common (breast cancer) | EXAMPLE: P | EXAMPLE: >90% are somatic; rare germline alterations associated with Li-Fraumeni syndrome (add reference). Denotes a poor prognosis in breast cancer. | |
| EXAMPLE: BRAF; Activating mutations | EXAMPLE: Activating mutations | EXAMPLE: Oncogene | EXAMPLE: Common (melanoma) | EXAMPLE: T | ||
Note: A more extensive list of mutations can be found in cBioportal, COSMIC, and/or other databases. When applicable, gene-specific pages within the CCGA site directly link to pertinent external content.
In a 2016 paper, it was shown that in the iAMP21-ALL exome, the mutations were more commonly transitions (for example: C>T) than transversions or indels.[4][7] Frequently, mutations in the RAS signaling pathway have been observed. Interestingly, these mutations were observed to coexist in patterns ranging from 2-3 mutated genes to 2-4 mutations in the same gene in one sample. Further, the FLT3-ITD was more prevalent in iAMP21-ALL.[7]
| Gene; Genetic Alteration | Presumed Mechanism (Tumor Suppressor Gene [TSG] / Oncogene / Other) | Prevalence (COSMIC / TCGA / Other) | Concomitant Mutations | Mutually Exclusive Mutations | Diagnostic Significance (Yes, No or Unknown) | Prognostic Significance (Yes, No or Unknown) | Therapeutic Significance (Yes, No or Unknown) | Notes |
|---|---|---|---|---|---|---|---|---|
| NRAS | EXAMPLE: TSG | 45% | EXAMPLE: IDH1 R123H | EXAMPLE: EGFR amplification | EXAMPLE: Excludes hairy cell leukemia (HCL) (add reference). | |||
| KRAS | 18% | |||||||
| FLT3 | 20% | |||||||
| PTPN11 | 11% | |||||||
| BRAF | 2% | |||||||
| NF1 | 2% |
Note: A more extensive list of mutations can be found in cBioportal (https://www.cbioportal.org/), COSMIC (https://cancer.sanger.ac.uk/cosmic), ICGC (https://dcc.icgc.org/) and/or other databases. When applicable, gene-specific pages within the CCGA site directly link to pertinent external content.
Epigenomic Alterations
N/A
Genes and Main Pathways Involved
Put your text here and fill in the table
| Gene; Genetic Alteration | Pathway | Pathophysiologic Outcome |
|---|---|---|
| RUNX1 | RAS pathway[7] | EXAMPLE: Increased cell growth and proliferation |
| EXAMPLE: CDKN2A; Inactivating mutations | EXAMPLE: Cell cycle regulation | EXAMPLE: Unregulated cell division |
| EXAMPLE: KMT2C and ARID1A; Inactivating mutations | EXAMPLE: Histone modification, chromatin remodeling | EXAMPLE: Abnormal gene expression program |
Genetic Diagnostic Testing Methods
Different methodologies are able to detect the iAMP21, such as:
- Fluorescence in situ hybridization (FISH) utilizing the probe set for the t(12;21)
- Conventional chromosome analysis
- Multiplex ligation-dependent probe amplification (MLPA)
- Chromosomal microarray (CMA)
- Next generation sequencing (NGS).
Familial Forms
N/A
Additional Information
This disease is defined/characterized as detailed below:
- Intrachromosomal amplification of chromosome 21 (iAMP21) is a neoplasm of lymphoblasts that are of the B-cell lineage. It is characterized by amplification of the RUNX1 gene at 21q22.3 on a structurally abnormal chromosome 21. Amplification is defined as ≥5 copies of RUNX1 detected by FISH or ≥3 copies of RUNX1 on a single abnormal chromosome 21.[8]
The epidemiology/prevalence of this disease is detailed below:
- iAMP21 is observed most often in the older pediatric group (median age of 9 years, with a range of 2-30 years). It accounts for ~2% of B-ALL cases including ~2% of standard-risk and 3% of high-risk patients. The incidence in adult B-ALL has not been established; however, it appears to be less prevalent than in the pediatric population.[4]
- Further, patients carrying a rob(15;21)(q10;q10) have an ~2700-fold increased risk of developing iAMP21 ALL compared to the general population. Additionally, patients with a constitutional ring chromosome 21, r(21), may potentially be predisposed to iAMP21 ALL.[4]
The clinical features of this disease are detailed below:
- ~50% of cases are classified as high-risk based on an age of ≥10 years.[9] Pediatric iAMP21 has been associated with a poor outcome. It displays an increased rate of relapse when treated on standard protocols. Further, the event-free survival and overall survival were significantly worse for individuals with the iAMP21 and standard-risk B-ALL, but not significant in individuals with iAMP21 and high-risk B-ALL.
- Laboratory findings - Low platelet count; Low WBC count (<50,000/μl)
The sites of involvement of this disease are detailed below:
- Bone Marrow and peripheral blood
The morphologic features of this disease are detailed below:
- There are no unique morphological or cytochemical features that distinguish this entity from other types of ALL.[8]
The immunophenotype of this disease is detailed below:
- No detailed information is known, other than these cases occur exclusively in B-ALL.[8]
Links
References
(use the "Cite" icon at the top of the page) (Instructions: Add each reference into the text above by clicking where you want to insert the reference, selecting the “Cite” icon at the top of the wiki page, and using the “Automatic” tab option to search by PMID to select the reference to insert. If a PMID is not available, such as for a book, please use the “Cite” icon, select “Manual” and then “Basic Form”, and include the entire reference. To insert the same reference again later in the page, select the “Cite” icon and “Re-use” to find the reference; DO NOT insert the same reference twice using the “Automatic” tab as it will be treated as two separate references. The reference list in this section will be automatically generated and sorted.)
- ↑ Harewood, L.; et al. (2003-03). "Amplification of AML1 on a duplicated chromosome 21 in acute lymphoblastic leukemia: a study of 20 cases". Leukemia. 17 (3): 547–553. doi:10.1038/sj.leu.2402849. ISSN 0887-6924. PMID 12646943. Check date values in:
|date=(help) - ↑ Arber, Daniel A. (04 2019). "The 2016 WHO classification of acute myeloid leukemia: What the practicing clinician needs to know". Seminars in Hematology. 56 (2): 90–95. doi:10.1053/j.seminhematol.2018.08.002. ISSN 1532-8686. PMID 30926096. Check date values in:
|date=(help) - ↑ Johnson, Ryan C.; et al. (2015-07). "Cytogenetic Variation of B-Lymphoblastic Leukemia With Intrachromosomal Amplification of Chromosome 21 (iAMP21): A Multi-Institutional Series Review". American Journal of Clinical Pathology. 144 (1): 103–112. doi:10.1309/AJCPLUYF11HQBYRB. ISSN 1943-7722. PMID 26071468. Check date values in:
|date=(help) - ↑ Jump up to: 4.0 4.1 4.2 4.3 Akkari, Yassmine M. N.; et al. (2020-05). "Evidence-based review of genomic aberrations in B-lymphoblastic leukemia/lymphoma: Report from the cancer genomics consortium working group for lymphoblastic leukemia". Cancer Genetics. 243: 52–72. doi:10.1016/j.cancergen.2020.03.001. ISSN 2210-7762. PMID 32302940 Check
|pmid=value (help). Check date values in:|date=(help) - ↑ Rand, Vikki; et al. (2011-06-23). "Genomic characterization implicates iAMP21 as a likely primary genetic event in childhood B-cell precursor acute lymphoblastic leukemia". Blood. 117 (25): 6848–6855. doi:10.1182/blood-2011-01-329961. ISSN 1528-0020. PMID 21527530.
- ↑ Hunger, Stephen P.; et al. (2012-05-10). "Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children's oncology group". Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 30 (14): 1663–1669. doi:10.1200/JCO.2011.37.8018. ISSN 1527-7755. PMC 3383113. PMID 22412151.
- ↑ Jump up to: 7.0 7.1 7.2 Ryan, S. L.; et al. (09 2016). "The role of the RAS pathway in iAMP21-ALL". Leukemia. 30 (9): 1824–1831. doi:10.1038/leu.2016.80. ISSN 1476-5551. PMC 5017527. PMID 27168466. Check date values in:
|date=(help) - ↑ Jump up to: 8.0 8.1 8.2 Borowitz MJ, et al., (2017). B-Lymphoblastic leukaemia/lymphoma with recurrent genetic abnormalities, in World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues, Revised 4th edition. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Arber DA, Hasserjian RP, Le Beau MM, Orazi A, and Siebert R, Editors. IARC Press: Lyon, France.
- ↑ Harrison, Christine J. (2015-02-26). "Blood Spotlight on iAMP21 acute lymphoblastic leukemia (ALL), a high-risk pediatric disease". Blood. 125 (9): 1383–1386. doi:10.1182/blood-2014-08-569228. ISSN 1528-0020. PMID 25608562.
Notes
*Primary authors will typically be those that initially create and complete the content of a page. If a subsequent user modifies the content and feels the effort put forth is of high enough significance to warrant listing in the authorship section, please contact the Associate Editor or other CCGA representative. When pages have a major update, the new author will be acknowledged at the beginning of the page, and those who contributed previously will be acknowledged below as a prior author.
Prior Author(s):
*Citation of this Page: “B-lymphoblastic leukaemia/lymphoma with iAMP21”. Compendium of Cancer Genome Aberrations (CCGA), Cancer Genomics Consortium (CGC), updated 03/24/2025, https://ccga.io/index.php/HAEM5:B-lymphoblastic_leukaemia/lymphoma_with_iAMP21.