GTS5:Neurofibromatosis type 1 (NF1)
Primary Author(s)*
Ngonidzashe Faya, PhD
Madina Sukhanova, PhD, FACMG
Cancer Category/Type
Genetic Tumour Syndromes (GTS)
Cancer Sub-Classification / Subtype
Put your text here
Definition / Description of Disease
Neurofibromatosis type 1(NF1) is an autosomal dominant disorder caused by a pathogenic NF1 variant, characterized by at least two of eight diagnostic criteria.
Essential and desirable diagnostic criteria
Essential:
A clinical diagnosis of NF1 requires the presence of at least two of the following features:
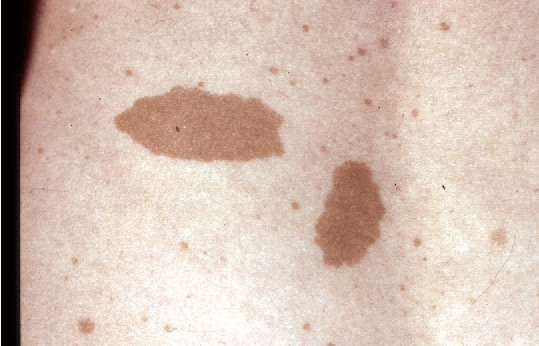
(1) Six or more café-au-lait macules (CALM) (> 5 mm diameter in children, > 15 mm in adults)
(2) Axillary/inguinal freckling
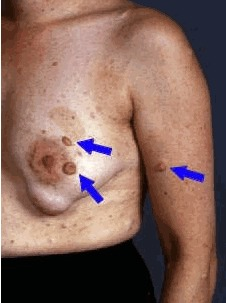
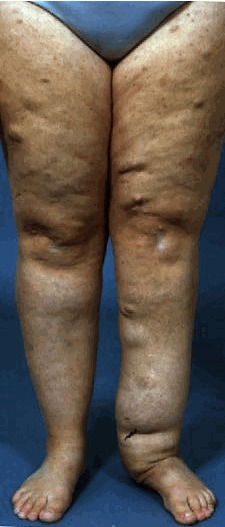
(3) Two or more neurofibromas of any type or one plexiform neurofibroma (PN)
(4) Optic pathway glioma (OPG)
(5) Two or more iris hamartomas (Lisch nodules) or two or more choroidal abnormalities
(6) Distinctive bony abnormality (anterolateral bowing of the tibia, pseudarthrosis of a long bone, sphenoid dysplasia)
(7) Heterozygous pathogenic NF1 variant with a variant allele fraction of 50% in apparently normal tissue
(8) Parent with NF1 (by the above criteria).
These criteria were revised in 2021[1]. Of note, there are other conditions with features that overlap with NF1, including Legius syndrome (OMIM 611431), Noonan syndrome[2], and constitutive mismatch repair deficiency[3].
Synonyms / Terminology
Not recommended: Von Recklinghausen disease; peripheral neurofibromatosis
Epidemiology / Prevalence
NF1 is a common autosomal dominant disorder with a birth incidence of 1 per 3000 [4]
Clinical Features
Multiple café-au-lait macules (CALMs) are typically present at birth and increase in number during early childhood. Axillary and inguinal freckling occurs in >80% and Lisch nodules (iris hamartomas) in >90% of adults with NF1. Cutaneous neurofibromas (>85% of adults with NF1) frequently develop during puberty[5], whereas plexiform neurofibromas (PN) are likely congenital, arising in 30–50% of children with NF1[6]. Individual PNs have variable growth rates, but exhibit the greatest growth during infancy and childhood. A subset of PNs transform into atypical neurofibroma/atypical neurofibromatous neoplasms of uncertain biological potential (ANNUBP), which can be premalignant lesions with increased risk of transformation into high-grade malignant peripheral nerve sheath tumours (MPNSTs)[7][8]. Possible ANNUBP or MPNST should be considered in patients with growing nodular PNs, PNs in which an isolated portion exhibits disproportionate growth, or adults with continued PN growth.
Specific NF1-associated bone abnormalities, including severe scoliosis, sphenoid wing dysplasia, non-ossifying fibromas, decreased bone mineral density, and congenital tibial bowing, are encountered. Progressive tibial bowing can result in pathological fracture and pseudarthrosis[9].
Many children exhibit learning disabilities, attention deficits, and problems with reciprocal social interactions[10]. Children with NF1 can also develop vasculopathies, especially of the renal and CNS circulation[11][12]. Macrocephaly, short stature, epilepsy, headache (including migraine), chronic pain, neuropathy and sleep disturbances are over-represented in people with NF1[13][14][15]. Fifteen percent of children will develop an optic pathway glioma/pilocytic astrocytoma (OPG), which causes vision loss in 30-50% of affected individuals[16]. Low-grade gliomas (LGG) are found in other locations, especially the brainstem[17][18]. Adults with NF1 are prone to malignant gliomas[19]. Brain MRI in children show focal areas of high signal intensity on T2-weighted sequences, which tend to disappear with age and are sometimes difficult to differentiate from LGGs[20], as well as larger grey matter and corpus callosum volumes[21]. Other neoplasms observed at increased frequency are juvenile myelomonocytic leukaemia, rhabdomyosarcoma, glomus tumours of the digits, gastrointestinal stromal tumours, phaeochromocytoma, breast cancer in females, and duodenal neuroendocrine tumours (somatostatinomas)[22].
| Signs and Symptoms | EXAMPLE Asymptomatic (incidental finding on complete blood counts)
EXAMPLE B-symptoms (weight loss, fever, night sweats) EXAMPLE Fatigue EXAMPLE Lymphadenopathy (uncommon) |
| Laboratory Findings | EXAMPLE Cytopenias
EXAMPLE Lymphocytosis (low level) |
Sites of Involvement
Put your text here
Morphologic Features
Immunophenotype
Put your text here and fill in the table
| Finding | Marker |
|---|---|
| Positive (universal) | EXAMPLE CD1 |
| Positive (subset) | EXAMPLE CD2 |
| Negative (universal) | EXAMPLE CD3 |
| Negative (subset) | EXAMPLE CD4 |
Chromosomal Rearrangements (Gene Fusions)
Put your text here and fill in the table
| Chromosomal Rearrangement | Genes in Fusion (5’ or 3’ Segments) | Pathogenic Derivative | Prevalence | Diagnostic Significance (Yes, No or Unknown) | Prognostic Significance (Yes, No or Unknown) | Therapeutic Significance (Yes, No or Unknown) | Notes |
|---|---|---|---|---|---|---|---|
| EXAMPLE t(9;22)(q34;q11.2) | EXAMPLE 3'ABL1 / 5'BCR | EXAMPLE der(22) | EXAMPLE 20% (COSMIC)
EXAMPLE 30% (add reference) |
Yes | No | Yes | EXAMPLE
The t(9;22) is diagnostic of CML in the appropriate morphology and clinical context (add reference). This fusion is responsive to targeted therapy such as Imatinib (Gleevec) (add reference). |
Individual Region Genomic Gain/Loss/LOH
Put your text here and fill in the table
| Chr # | Gain / Loss / Amp / LOH | Minimal Region Genomic Coordinates [Genome Build] | Minimal Region Cytoband | Diagnostic Significance (Yes, No or Unknown) | Prognostic Significance (Yes, No or Unknown) | Therapeutic Significance (Yes, No or Unknown) | Notes |
|---|---|---|---|---|---|---|---|
| EXAMPLE
7 |
EXAMPLE Loss | EXAMPLE
chr7:1- 159,335,973 [hg38] |
EXAMPLE
chr7 |
Yes | Yes | No | EXAMPLE
Presence of monosomy 7 (or 7q deletion) is sufficient for a diagnosis of AML with MDS-related changes when there is ≥20% blasts and no prior therapy (add reference). Monosomy 7/7q deletion is associated with a poor prognosis in AML (add reference). |
| EXAMPLE
8 |
EXAMPLE Gain | EXAMPLE
chr8:1-145,138,636 [hg38] |
EXAMPLE
chr8 |
No | No | No | EXAMPLE
Common recurrent secondary finding for t(8;21) (add reference). |
Characteristic Chromosomal Patterns
Put your text here
| Chromosomal Pattern | Diagnostic Significance (Yes, No or Unknown) | Prognostic Significance (Yes, No or Unknown) | Therapeutic Significance (Yes, No or Unknown) | Notes |
|---|---|---|---|---|
| EXAMPLE
Co-deletion of 1p and 18q |
Yes | No | No | EXAMPLE:
See chromosomal rearrangements table as this pattern is due to an unbalanced derivative translocation associated with oligodendroglioma (add reference). |
Gene Mutations (SNV/INDEL)
Put your text here and fill in the table
| Gene; Genetic Alteration | Presumed Mechanism (Tumor Suppressor Gene [TSG] / Oncogene / Other) | Prevalence (COSMIC / TCGA / Other) | Concomitant Mutations | Mutually Exclusive Mutations | Diagnostic Significance (Yes, No or Unknown) | Prognostic Significance (Yes, No or Unknown) | Therapeutic Significance (Yes, No or Unknown) | Notes |
|---|---|---|---|---|---|---|---|---|
| EXAMPLE: TP53; Variable LOF mutations
EXAMPLE: EGFR; Exon 20 mutations EXAMPLE: BRAF; Activating mutations |
EXAMPLE: TSG | EXAMPLE: 20% (COSMIC)
EXAMPLE: 30% (add Reference) |
EXAMPLE: IDH1 R123H | EXAMPLE: EGFR amplification | EXAMPLE: Excludes hairy cell leukemia (HCL) (add reference).
|
Note: A more extensive list of mutations can be found in cBioportal (https://www.cbioportal.org/), COSMIC (https://cancer.sanger.ac.uk/cosmic), ICGC (https://dcc.icgc.org/) and/or other databases. When applicable, gene-specific pages within the CCGA site directly link to pertinent external content.
Epigenomic Alterations
Put your text here
Genes and Main Pathways Involved
Put your text here and fill in the table
| Gene; Genetic Alteration | Pathway | Pathophysiologic Outcome |
|---|---|---|
| EXAMPLE: BRAF and MAP2K1; Activating mutations | EXAMPLE: MAPK signaling | EXAMPLE: Increased cell growth and proliferation |
| EXAMPLE: CDKN2A; Inactivating mutations | EXAMPLE: Cell cycle regulation | EXAMPLE: Unregulated cell division |
| EXAMPLE: KMT2C and ARID1A; Inactivating mutations | EXAMPLE: Histone modification, chromatin remodeling | EXAMPLE: Abnormal gene expression program |
Genetic Diagnostic Testing Methods
Put your text here
Familial Forms
Put your text here
Additional Information
Put your text here
Links
Put your text placeholder here (use "Link" icon at top of page)
References
- ↑ Legius, Eric; et al. (2021-08). "Revised diagnostic criteria for neurofibromatosis type 1 and Legius syndrome: an international consensus recommendation". Genetics in Medicine: Official Journal of the American College of Medical Genetics. 23 (8): 1506–1513. doi:10.1038/s41436-021-01170-5. ISSN 1530-0366. PMC 8354850 Check
|pmc=value (help). PMID 34012067 Check|pmid=value (help). Check date values in:|date=(help) - ↑ Conboy, Erin; et al. (2016-02). "Paraspinal neurofibromas and hypertrophic neuropathy in Noonan syndrome with multiple lentigines". Journal of Medical Genetics. 53 (2): 123–126. doi:10.1136/jmedgenet-2015-103177. ISSN 1468-6244. PMID 26337637. Check date values in:
|date=(help) - ↑ M, Suerink; et al. (2019 Feb). "Constitutional mismatch repair deficiency as a differential diagnosis of neurofibromatosis type 1: consensus guidelines for testing a child without malignancy". Journal of medical genetics. 56 (2). doi:10.1136/jmedgenet-2018-105664. ISSN 1468-6244. PMID 30415209. Check date values in:
|date=(help) - ↑ Uusitalo, Elina; et al. (2015-03). "Incidence and mortality of neurofibromatosis: a total population study in Finland". The Journal of Investigative Dermatology. 135 (3): 904–906. doi:10.1038/jid.2014.465. ISSN 1523-1747. PMID 25354145. Check date values in:
|date=(help) - ↑ Gutmann, David H.; et al. (2017-02-23). "Neurofibromatosis type 1". Nature Reviews. Disease Primers. 3: 17004. doi:10.1038/nrdp.2017.4. ISSN 2056-676X. PMID 28230061.
- ↑ Gutmann, David H.; et al. (2017-02-23). "Neurofibromatosis type 1". Nature Reviews. Disease Primers. 3: 17004. doi:10.1038/nrdp.2017.4. ISSN 2056-676X. PMID 28230061.
- ↑ Miettinen, Markku M.; et al. (2017-09). "Histopathologic evaluation of atypical neurofibromatous tumors and their transformation into malignant peripheral nerve sheath tumor in patients with neurofibromatosis 1-a consensus overview". Human Pathology. 67: 1–10. doi:10.1016/j.humpath.2017.05.010. ISSN 1532-8392. PMC 5628119. PMID 28551330. Check date values in:
|date=(help) - ↑ Beert, Eline; et al. (2011-12). "Atypical neurofibromas in neurofibromatosis type 1 are premalignant tumors". Genes, Chromosomes & Cancer. 50 (12): 1021–1032. doi:10.1002/gcc.20921. ISSN 1098-2264. PMID 21987445. Check date values in:
|date=(help) - ↑ Schindeler, Aaron; et al. (2008-04). "Recent insights into bone development, homeostasis, and repair in type 1 neurofibromatosis (NF1)". Bone. 42 (4): 616–622. doi:10.1016/j.bone.2007.11.006. ISSN 8756-3282. PMID 18248783. Check date values in:
|date=(help) - ↑ Morris, Stephanie M.; et al. (2016-12-01). "Disease Burden and Symptom Structure of Autism in Neurofibromatosis Type 1: A Study of the International NF1-ASD Consortium Team (INFACT)". JAMA psychiatry. 73 (12): 1276–1284. doi:10.1001/jamapsychiatry.2016.2600. ISSN 2168-6238. PMC 5298203. PMID 27760236.
- ↑ Celik, Binnaz; et al. (2021-12). "Renal manifestations in children with neurofibromatosis type 1". European Journal of Pediatrics. 180 (12): 3477–3482. doi:10.1007/s00431-021-04144-6. ISSN 1432-1076. PMID 34091747 Check
|pmid=value (help). Check date values in:|date=(help) - ↑ Brosius, Stephanie N.; et al. (2022-09). "Characteristics of Moyamoya Syndrome in Pediatric Patients With Neurofibromatosis Type 1". Pediatric Neurology. 134: 85–92. doi:10.1016/j.pediatrneurol.2022.05.013. ISSN 1873-5150. PMID 35849956 Check
|pmid=value (help). Check date values in:|date=(help) - ↑ Licis, Amy K.; et al. (2013-11). "Prevalence of Sleep Disturbances in Children With Neurofibromatosis Type 1". Journal of Child Neurology. 28 (11): 1400–1405. doi:10.1177/0883073813500849. ISSN 1708-8283. PMC 3805763. PMID 24065580. Check date values in:
|date=(help) - ↑ Stewart, Douglas R.; et al. (2018-07). "Care of adults with neurofibromatosis type 1: a clinical practice resource of the American College of Medical Genetics and Genomics (ACMG)". Genetics in Medicine: Official Journal of the American College of Medical Genetics. 20 (7): 671–682. doi:10.1038/gim.2018.28. ISSN 1530-0366. PMID 30006586. Check date values in:
|date=(help) - ↑ Miller, David T.; et al. (2019-05). "Health Supervision for Children With Neurofibromatosis Type 1". Pediatrics. 143 (5): e20190660. doi:10.1542/peds.2019-0660. ISSN 1098-4275. PMID 31010905. Check date values in:
|date=(help) - ↑ de Blank, Peter M. K.; et al. (2017-09). "Optic Pathway Gliomas in Neurofibromatosis Type 1: An Update: Surveillance, Treatment Indications, and Biomarkers of Vision". Journal of Neuro-Ophthalmology: The Official Journal of the North American Neuro-Ophthalmology Society. 37 Suppl 1 (Suppl 1): S23–S32. doi:10.1097/WNO.0000000000000550. ISSN 1536-5166. PMC 7410089 Check
|pmc=value (help). PMID 28806346. Check date values in:|date=(help) - ↑ Mahdi, Jasia; et al. (2020-08-25). "Nonoptic pathway tumors in children with neurofibromatosis type 1". Neurology. 95 (8): e1052–e1059. doi:10.1212/WNL.0000000000009458. ISSN 1526-632X. PMC 7668552 Check
|pmc=value (help). PMID 32300062 Check|pmid=value (help). - ↑ Mahdi, Jasia; et al. (2020-08-25). "Nonoptic pathway tumors in children with neurofibromatosis type 1". Neurology. 95 (8): e1052–e1059. doi:10.1212/WNL.0000000000009458. ISSN 1526-632X. PMC 7668552 Check
|pmc=value (help). PMID 32300062 Check|pmid=value (help). - ↑ Packer, Roger J.; et al. (2020-06-09). "Implications of new understandings of gliomas in children and adults with NF1: report of a consensus conference". Neuro-Oncology. 22 (6): 773–784. doi:10.1093/neuonc/noaa036. ISSN 1523-5866. PMC 7283027 Check
|pmc=value (help). PMID 32055852 Check|pmid=value (help). - ↑ Griffith, Jennifer L.; et al. (2018-08). "Increased prevalence of brain tumors classified as T2 hyperintensities in neurofibromatosis 1". Neurology. Clinical Practice. 8 (4): 283–291. doi:10.1212/CPJ.0000000000000494. ISSN 2163-0402. PMC 6105062. PMID 30140579. Check date values in:
|date=(help) - ↑ Moore, B. D.; et al. (2000-02-22). "Brain volume in children with neurofibromatosis type 1: relation to neuropsychological status". Neurology. 54 (4): 914–920. doi:10.1212/wnl.54.4.914. ISSN 0028-3878. PMID 10690986.
- ↑ Brems, Hilde; et al. (2009-05). "Mechanisms in the pathogenesis of malignant tumours in neurofibromatosis type 1". The Lancet. Oncology. 10 (5): 508–515. doi:10.1016/S1470-2045(09)70033-6. ISSN 1474-5488. PMID 19410195. Check date values in:
|date=(help) - ↑ Friedman, Jan M. (2022-04-21). "Figure 1. [Café au lait macules]".
- ↑ Friedman, Jan M. (2022-04-21). "Figure 2. [Neurofibromas]".
- ↑ Friedman, Jan M. (2022-04-21). "Figure 3. [Plexiform neurofibroma]".
(use "Cite" icon at top of page)
EXAMPLE Book
- Arber DA, et al., (2017). Acute myeloid leukaemia with recurrent genetic abnormalities, in World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues, Revised 4th edition. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Arber DA, Hasserjian RP, Le Beau MM, Orazi A, and Siebert R, Editors. IARC Press: Lyon, France, p129-171.
Notes
*Primary authors will typically be those that initially create and complete the content of a page. If a subsequent user modifies the content and feels the effort put forth is of high enough significance to warrant listing in the authorship section, please contact the CCGA coordinators (contact information provided on the homepage). Additional global feedback or concerns are also welcome.