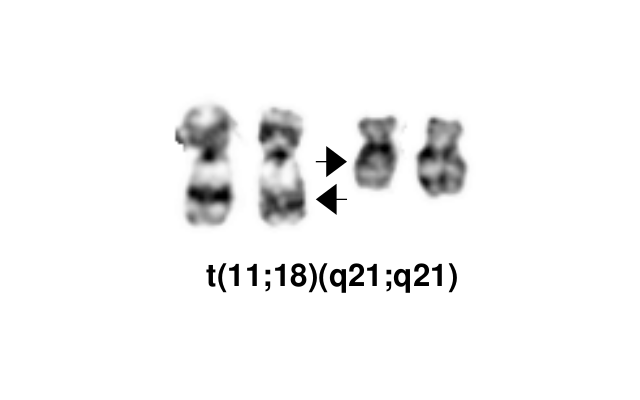Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue
Haematolymphoid Tumours (WHO Classification, 5th ed.)
| This page is under construction |
editContent Update To WHO 5th Edition Classification Is In Process; Content Below is Based on WHO 4th Edition ClassificationThis page was converted to the new template on 2023-12-07. The original page can be found at HAEM4:Extranodal Marginal Zone Lymphoma of Mucosa-Associated Lymphoid Tissue (MALT Lymphoma).
(General Instructions – The main focus of these pages is the clinically significant genetic alterations in each disease type. Use HUGO-approved gene names and symbols (italicized when appropriate), HGVS-based nomenclature for variants, as well as generic names of drugs and testing platforms or assays if applicable. Please complete tables whenever possible and do not delete them (add N/A if not applicable in the table and delete the examples); to add (or move) a row or column to a table, click within the table and select the > symbol that appears to be given options. Please do not delete or alter the section headings. The use of bullet points alongside short blocks of text rather than only large paragraphs is encouraged. Additional instructions below in italicized blue text should not be included in the final page content. Please also see Author_Instructions and FAQs as well as contact your Associate Editor or Technical Support)
Primary Author(s)*
WHO Classification of Disease
| Structure | Disease |
|---|---|
| Book | Haematolymphoid Tumours (5th ed.) |
| Category | B-cell lymphoid proliferations and lymphomas |
| Family | Mature B-cell neoplasms |
| Type | Marginal zone lymphoma |
| Subtype(s) | Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue |
Definition / Description of Disease
- Extranodal lymphoma composed of morphologically heterogeneous small B cells, including marginal zone (centrocyte-like) cells, monocytoid cells, small lymphocytes, and scattered immunoblasts and centroblast-like cells [1]. Plasmacytic differentiation present in some cases.
- Infectious, chronic inflammatory, or autoimmune conditions are implicated in pathogenesis of MALT lymphoma.
- Clinically indolent (median survival 8 yrs), transformation to DLBCL occurs <10% cases [2].
Synonyms / Terminology
MALToma
Epidemiology / Prevalence
- 7-8% of all B cell lymphoma [3]
- Group of Marginal zone lymphomas: HAEM5:Nodal marginal zone lymphoma, HAEM5:Splenic marginal zone lymphoma, MALT lymphoma
- 50% of primary gastric lymphoma [4][5]
- Median age 70th decade, F:M=1:1 with site specific sex differences; female predominance in thyroid and salivary glands [6]
Clinical Features
Put your text here and fill in the table (Instruction: Can include references in the table. Do not delete table.)
| Signs and Symptoms | EXAMPLE: Asymptomatic (incidental finding on complete blood counts)
EXAMPLE: B-symptoms (weight loss, fever, night sweats) EXAMPLE: Fatigue EXAMPLE: Lymphadenopathy (uncommon) |
| Laboratory Findings | EXAMPLE: Cytopenias
EXAMPLE: Lymphocytosis (low level) |
editv4:Clinical FeaturesThe content below was from the previous version of the page. Please incorporate above.
- Site specific:
- Stomach:
- Can be asymptomatic or cause epigastric pain, anorexia, weight loss, anemia, early satiety, occasionally fever and night sweat
- Associated with Helicobacter pylori infection (32% - >90%)[7][8][9]
- > 75% of cases respond to H. pylori eradication with regression of lymphoma
- H. pylori eradication therapy is 1st-line treatment; Chemotherapy ± radiation if DLBCL transformation or disseminated
- Cases with t(11;18)(q21;q21) are resistant to H. pylori eradication therapy
- 5-year overall survival ~ 90%
- Other organs: Mass and associated symptoms, higher chance of multiple site involvement and bone marrow involvement than gastric MALT lymphoma
- Small bowel: Associated with Campylobacter jejuni infection in patients with α-heavy chain disease
- Ocular adnexa: Associated with Chlamydia psittaci
- Skin: Associated with Borrelia burgdorferi
- Lung: Associated with Sjögren syndrome, cough and dyspnea (ref)
- Salivary glands: Associated with Sjögren syndrome
- Thyroid: Associated with Hashimoto thyroiditis
- Most are low stage (stage I and II) at presentation, with up to 40% have disease with multiple sites involvement and up to 20% of patients have bone marrow involvement, and both are associated with non-gastric MALT lymphoma
- Disseminated disease is usually refractory to chemotherapy
- Recurrences may involve other extranodal sites
- Serum paraprotein can be detected in one third of patients, particularly those with plasmacytic differentiation [10]. May be associated with Waldenström macroglobulinemia[11]
Sites of Involvement
- Localized disease (most patients)
- Stomach (35%, m/c)
- Eyes and ocular adnexa (13%)
- Lung (9%)
- Skin (9%)
- Salivary glands (8%)
- Breasts (3%)
- Thyroid (2%)
- Bone marrow involvement in minority of patients (2-20%)
- Multiple extranodal sites involvement
- Higher in non-gastric MALT lymphoma cases
- Generalized nodal involvement is rare (<10%)
Morphologic Features
- Lymphoepithelial lesion
- Specific but not sensitive, most prominent in thyroid and parotid glands
- Can be present in some reactive conditions or in other lymphoma subtype
- Infiltration and distortion of epithelial structures/glands (> 3 centrocyte-like cells causing epithelial damage)
- Epithelial degeneration and glandular structure destruction
- Specific but not sensitive, most prominent in thyroid and parotid glands
- Diffuse or nodular pattern of growth
- Expansion of marginal zone by cytologically heterogeneous cell population
- Predominantly atypical centrocyte-like cells with small, cleaved, irregular nuclei with abundant cytoplasm
- Monocytoid appearance with distinct rim of clear cytoplasm
- Scattered large cells (centroblasts or immunoblasts) are present; up to 10% of all cells
- Plasmacytic differentiation ± Dutcher bodies or Russell bodies (m/c in thyroid lymphoma)
- Amyloid deposition
- Expansion of marginal zone by cytologically heterogeneous cell population
- Transformation to diffuse large B-cell lymphoma
- Large cells form sheets or large clusters of > 20 cells
- May coexist with MALT lymphoma at initial presentation
- Multifocal disease
- ~ 25% of patients have > 1 extranodal site of involvement
- Large cells form sheets or large clusters of > 20 cells
Immunophenotype
| Finding | Marker |
|---|---|
| Positive (universal) | B cell associated markers (CD19, CD20, CD22, CD79a, PAX5) |
| Positive (subset) | Surface Ig
Cytoplasmic Ig (plasma cell component) Kappa/Lambda light chain restriction (plasma cell component) Bcl10 MNDA: MALT (61 - 75%) versus follicular (< 10%) lymphoma[12] IRTA1: potential MALT specific antibody [12] |
| Negative (universal) | IgD
CD5 CD10 CD23 BCL6 Bcl 1/Cyclin D1 |
| Variable | CD43
CD21, CD23, CD35: Disrupted and expanded meshwork of follicular dendritic cells) BCL2 CD11c |
| Miscellaneous | Keratin: can accentuate lymphoepithelial lesions
Congo red: can highlight amyloid deposition present in a subset of cases |
Chromosomal Rearrangements (Gene Fusions)
Put your text here and fill in the table
| Chromosomal Rearrangement | Genes in Fusion (5’ or 3’ Segments) | Pathogenic Derivative | Prevalence | Diagnostic Significance (Yes, No or Unknown) | Prognostic Significance (Yes, No or Unknown) | Therapeutic Significance (Yes, No or Unknown) | Notes |
|---|---|---|---|---|---|---|---|
| EXAMPLE: t(9;22)(q34;q11.2) | EXAMPLE: 3'ABL1 / 5'BCR | EXAMPLE: der(22) | EXAMPLE: 20% (COSMIC)
EXAMPLE: 30% (add reference) |
Yes | No | Yes | EXAMPLE:
The t(9;22) is diagnostic of CML in the appropriate morphology and clinical context (add reference). This fusion is responsive to targeted therapy such as Imatinib (Gleevec) (add reference). |
editv4:Chromosomal Rearrangements (Gene Fusions)The content below was from the previous version of the page. Please incorporate above.
Chromosomal Rearrangement Genes in Fusion (5’ or 3’ Segments)
Dysregulated pathway Prevalence Anatomic Sites Notes t(11;18)(q21;q21) 5'BIRC3 (API2)-3'MALT1 NF-κB 15-40% Stomach, lung, ocular adnexa Associated with resistance to H. pylori eradication therapy t(14;18)(q32;q21) IGH-MALT1 NF-κB 10-15% Ocular adnexa, skin, liver, salivary gland Non-gastric MALT lymphoma Associated with other karyotype abnormalities (trisomy)
t(14;18)(q32;q21) IGH-MALT1 is indistinguishable from IgH / BCL2 by cytogenetics.
t(3;14)(p13;q32) FOXP1-IGH Wnt ~5% Thyroid, ocular adnexa, skin not seen in gastric cases. Transcriptional deregulation of FOXP1 t(1;14)(p22:q32) BCL10-IGH NF-κB ~5% Lung, small intestine not seen in gastric cases. Transcriptional deregulation of BCL10 t(X;14)(p11.2;q32) GPR34-IGH NF-κB? ~2% Lung, parotid gland t(1;2)(p22;q12) BCL10-IGK Unclear ~1% Stomach t(1;14)(p21;q32) CNN3-IGH NF-κB Parotid gland t(9;14)(p24;q32) KDM4C-IGH Chromatin remodeling[13] Parotid gland, ocular adnexa t(5;14)(q34;q32) TENM2-IGH Unclear Skin, ocular adnexa t(6;7)(q25;q11) Unknown Unclear Ocular adnexa
editUnassigned ReferencesThe following referenees were placed in the header. Please place them into the appropriate locations in the text.
Individual Region Genomic Gain / Loss / LOH
Put your text here and fill in the table (Instructions: Includes aberrations not involving gene fusions. Can include references in the table. Can refer to CGC workgroup tables as linked on the homepage if applicable. Do not delete table.)
| Chr # | Gain / Loss / Amp / LOH | Minimal Region Genomic Coordinates [Genome Build] | Minimal Region Cytoband | Diagnostic Significance (Yes, No or Unknown) | Prognostic Significance (Yes, No or Unknown) | Therapeutic Significance (Yes, No or Unknown) | Notes |
|---|---|---|---|---|---|---|---|
| EXAMPLE:
7 |
EXAMPLE: Loss | EXAMPLE:
chr7:1- 159,335,973 [hg38] |
EXAMPLE:
chr7 |
Yes | Yes | No | EXAMPLE:
Presence of monosomy 7 (or 7q deletion) is sufficient for a diagnosis of AML with MDS-related changes when there is ≥20% blasts and no prior therapy (add reference). Monosomy 7/7q deletion is associated with a poor prognosis in AML (add reference). |
| EXAMPLE:
8 |
EXAMPLE: Gain | EXAMPLE:
chr8:1-145,138,636 [hg38] |
EXAMPLE:
chr8 |
No | No | No | EXAMPLE:
Common recurrent secondary finding for t(8;21) (add reference). |
editv4:Individual Region Genomic Gain / Loss / LOHThe content below was from the previous version of the page. Please incorporate above.
Chr # Gain / Loss / Amp / LOH Region Possible Target Genes Frequency Notes 3 Gain 3q NFKBIZ, BCL6 30% often associated with other translocations 6 Loss 6q23 TNFAIP3/A20 30% often associated with other translocations 3 Gain 3p FOXP1 26% often associated with other translocations 6 Gain 6p25.1-p21.32 unknown unknown 18 Gain 18q unknown unknown common secondary finding in B-cell neoplasm
editUnassigned ReferencesThe following referenees were placed in the header. Please place them into the appropriate locations in the text.
Characteristic Chromosomal Patterns
Put your text here (EXAMPLE PATTERNS: hyperdiploid; gain of odd number chromosomes including typically chromosome 1, 3, 5, 7, 11, and 17; co-deletion of 1p and 19q; complex karyotypes without characteristic genetic findings; chromothripsis. Do not delete table.)
| Chromosomal Pattern | Diagnostic Significance (Yes, No or Unknown) | Prognostic Significance (Yes, No or Unknown) | Therapeutic Significance (Yes, No or Unknown) | Notes |
|---|---|---|---|---|
| EXAMPLE:
Co-deletion of 1p and 18q |
Yes | No | No | EXAMPLE:
See chromosomal rearrangements table as this pattern is due to an unbalanced derivative translocation associated with oligodendroglioma (add reference). |
Gene Mutations (SNV / INDEL)
Put your text here and fill in the table (Instructions: This table is not meant to be an exhaustive list; please include only genes/alterations that are recurrent and common as well as either disease defining and/or clinically significant. Can include references in the table. For clinical significance, denote associations with FDA-approved therapy (not an extensive list of applicable drugs) and NCCN or other national guidelines if applicable. Can also refer to CGC workgroup tables as linked on the homepage if applicable as well as any high impact papers or reviews of gene mutations in this entity. Do not delete table.)
| Gene; Genetic Alteration | Presumed Mechanism (Tumor Suppressor Gene [TSG] / Oncogene / Other) | Prevalence (COSMIC / TCGA / Other) | Concomitant Mutations | Mutually Exclusive Mutations | Diagnostic Significance (Yes, No or Unknown) | Prognostic Significance (Yes, No or Unknown) | Therapeutic Significance (Yes, No or Unknown) | Notes |
|---|---|---|---|---|---|---|---|---|
| EXAMPLE: TP53; Variable LOF mutations
EXAMPLE: EGFR; Exon 20 mutations EXAMPLE: BRAF; Activating mutations |
EXAMPLE: TSG | EXAMPLE: 20% (COSMIC)
EXAMPLE: 30% (add Reference) |
EXAMPLE: IDH1 R123H | EXAMPLE: EGFR amplification | EXAMPLE: Excludes hairy cell leukemia (HCL) (add reference).
|
Note: A more extensive list of mutations can be found in cBioportal (https://www.cbioportal.org/), COSMIC (https://cancer.sanger.ac.uk/cosmic), ICGC (https://dcc.icgc.org/) and/or other databases. When applicable, gene-specific pages within the CCGA site directly link to pertinent external content.
editv4:Gene Mutations (SNV / INDEL)The content below was from the previous version of the page. Please incorporate above.
- MALT lymphoma presents somatically mutated immunoglobulin heavy chain variable region (IGHV) genes in nearly all cases. Site specific.[18]
- IGHVH1-69 in salivary gland lymphomas
- IGHVH3-30 or IGHVH3-23 in gastric MALT lymphomas responsive to H pylori eradication and without the t(11;18) translocation
- IGHVH4-34 in orbital adnexal lymphomas
- IGHV3 and IGHV4 families in pulmonary lymphomas
- IGHVH1-69 or IGHVH4-59 in cutaneous lymphomas
Gene; Genetic Alteration Presumed Mechanism (Tumor Suppressor Gene [TSG] / Oncogene / Other)
Prevalence Notes TNFAIP3 NF-κB negative regulator 29% Mutations includes deletions, SNVs, and promoter methylation, can be present in cases lacking specific translocations listed above. Mutations can be also found in many types of NHL. CREBBP Tumor Suppressor 22% KMT2C Tumor Suppressor 19% TET2 Myelopoiesis 17% SPEN 17% KMT2D Histone modification 15% LRP1B 15% PRDM1 15% TNFRSF14 NF-κB negative regulator 11% NOTCH1/NOTCH2 NOTCH signaling pathway 11% PIM1 cMyc Oncogene P53 Tumor Suppressor MYD88 6-9% L265P Note: A more extensive list of mutations can be found in cBioportal (https://www.cbioportal.org/), COSMIC (https://cancer.sanger.ac.uk/cosmic), ICGC (https://dcc.icgc.org/) and/or other databases. When applicable, gene-specific pages within the CCGA site directly link to pertinent external content.
editUnassigned ReferencesThe following referenees were placed in the header. Please place them into the appropriate locations in the text.
Epigenomic Alterations
N/A
Genes and Main Pathways Involved
Put your text here and fill in the table
| Gene; Genetic Alteration | Pathway | Pathophysiologic Outcome |
|---|---|---|
| EXAMPLE: BRAF and MAP2K1; Activating mutations | EXAMPLE: MAPK signaling | EXAMPLE: Increased cell growth and proliferation |
| EXAMPLE: CDKN2A; Inactivating mutations | EXAMPLE: Cell cycle regulation | EXAMPLE: Unregulated cell division |
| EXAMPLE: KMT2C and ARID1A; Inactivating mutations | EXAMPLE: Histone modification, chromatin remodeling | EXAMPLE: Abnormal gene expression program |
Genetic Diagnostic Testing Methods
Put your text here
Familial Forms
No familial predisposition for MALT lymphomas has been reported.
Additional Information
N/A
Links
HAEM5:Nodal marginal zone lymphoma
HAEM5:Splenic marginal zone lymphoma
References
- ↑ Swerdlow, SH. et al., (2017) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. International Agency for Research on Cancer (p 159-262).
- ↑ Deutsch, Alexander J. A.; et al. (2013-02). "Chemokine receptors in gastric MALT lymphoma: loss of CXCR4 and upregulation of CXCR7 is associated with progression to diffuse large B-cell lymphoma". Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc. 26 (2): 182–194. doi:10.1038/modpathol.2012.134. ISSN 1530-0285. PMID 22936065. Check date values in:
|date=(help) - ↑ Kuper-Hommel, Marion J. J.; et al. (2013-09). "Trends in incidence, therapy and outcome of localized nodal and extranodal marginal zone lymphomas: declining incidence and inferior outcome for gastrointestinal sites". Leukemia & Lymphoma. 54 (9): 1891–1897. doi:10.3109/10428194.2013.764421. ISSN 1029-2403. PMID 23302044. Check date values in:
|date=(help) - ↑ Doglioni, C.; et al. (1992-04-04). "High incidence of primary gastric lymphoma in northeastern Italy". Lancet (London, England). 339 (8797): 834–835. doi:10.1016/0140-6736(92)90280-g. ISSN 0140-6736. PMID 1347858.
- ↑ Isaacson, Peter G.; et al. (2004-08). "MALT lymphoma: from morphology to molecules". Nature Reviews. Cancer. 4 (8): 644–653. doi:10.1038/nrc1409. ISSN 1474-175X. PMID 15286744. Check date values in:
|date=(help) - ↑ "A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project". Blood. 89 (11): 3909–3918. 1997-06-01. ISSN 0006-4971. PMID 9166827.
- ↑ Wotherspoon, A. C.; et al. (1993-09-04). "Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori". Lancet (London, England). 342 (8871): 575–577. doi:10.1016/0140-6736(93)91409-f. ISSN 0140-6736. PMID 8102719.
- ↑ Luminari, S.; et al. (2010-04). "Decreasing incidence of gastric MALT lymphomas in the era of anti-Helicobacter pylori interventions: results from a population-based study on extranodal marginal zone lymphomas". Annals of Oncology: Official Journal of the European Society for Medical Oncology. 21 (4): 855–859. doi:10.1093/annonc/mdp402. ISSN 1569-8041. PMID 19850642. Check date values in:
|date=(help) - ↑ Sena Teixeira Mendes, Larissa; et al. (2014-09). "Helicobacter pylori infection in gastric extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) lymphoma: a re-evaluation". Gut. 63 (9): 1526–1527. doi:10.1136/gutjnl-2014-307389. ISSN 1468-3288. PMID 24951256. Check date values in:
|date=(help) - ↑ Wöhrer, Stefan; et al. (2004-11-01). "Monoclonal immunoglobulin production is a frequent event in patients with mucosa-associated lymphoid tissue lymphoma". Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 10 (21): 7179–7181. doi:10.1158/1078-0432.CCR-04-0803. ISSN 1078-0432. PMID 15534090.
- ↑ Valdez, R.; et al. (2001-11). "Waldenström macroglobulinemia caused by extranodal marginal zone B-cell lymphoma: a report of six cases". American Journal of Clinical Pathology. 116 (5): 683–690. doi:10.1309/6WPX-66CM-KGRH-V4RW. ISSN 0002-9173. PMID 11710684. Check date values in:
|date=(help) - ↑ 12.0 12.1 Wang, Zhen; et al. (2019-02-04). "IRTA1 and MNDA Expression in Marginal Zone Lymphoma: Utility in Differential Diagnosis and Implications for Classification". American Journal of Clinical Pathology. 151 (3): 337–343. doi:10.1093/ajcp/aqy144. ISSN 1943-7722. PMID 30346478.
- ↑ Rui, Lixin; et al. (2010-12-14). "Cooperative epigenetic modulation by cancer amplicon genes". Cancer Cell. 18 (6): 590–605. doi:10.1016/j.ccr.2010.11.013. ISSN 1878-3686. PMC 3049192. PMID 21156283.
- ↑ 14.0 14.1 Troppan, Katharina; et al. (2015). "Molecular Pathogenesis of MALT Lymphoma". Gastroenterology Research and Practice. 2015: 102656. doi:10.1155/2015/102656. ISSN 1687-6121. PMC 4397421. PMID 25922601.
- ↑ Rinaldi, Andrea; et al. (2011-02-03). "Genome-wide DNA profiling of marginal zone lymphomas identifies subtype-specific lesions with an impact on the clinical outcome". Blood. 117 (5): 1595–1604. doi:10.1182/blood-2010-01-264275. ISSN 1528-0020. PMID 21115979.
- ↑ Zucca, Emanuele; et al. (2016-04-28). "The spectrum of MALT lymphoma at different sites: biological and therapeutic relevance". Blood. 127 (17): 2082–2092. doi:10.1182/blood-2015-12-624304. ISSN 1528-0020. PMID 26989205.
- ↑ 17.0 17.1 Cascione, Luciano; et al. (2019-12). "Novel insights into the genetics and epigenetics of MALT lymphoma unveiled by next generation sequencing analyses". Haematologica. 104 (12): e558–e561. doi:10.3324/haematol.2018.214957. ISSN 1592-8721. PMC 6959164. PMID 31018978. Check date values in:
|date=(help) - ↑ Thieblemont, Catherine; et al. (2014-02). "Chronic inflammation and extra-nodal marginal-zone lymphomas of MALT-type". Seminars in Cancer Biology. 24: 33–42. doi:10.1016/j.semcancer.2013.11.005. ISSN 1096-3650. PMID 24333758. Check date values in:
|date=(help)
(use "Cite" icon at top of page)
Notes
*Primary authors will typically be those that initially create and complete the content of a page. If a subsequent user modifies the content and feels the effort put forth is of high enough significance to warrant listing in the authorship section, please contact the CCGA coordinators (contact information provided on the homepage). Additional global feedback or concerns are also welcome.
*Citation of this Page: “Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue”. Compendium of Cancer Genome Aberrations (CCGA), Cancer Genomics Consortium (CGC), updated 09/6/2024, https://ccga.io/index.php/HAEM5:Extranodal_marginal_zone_lymphoma_of_mucosa-associated_lymphoid_tissue.