Myeloid/Lymphoid Neoplasms with PDGFRA Rearrangement
editPREVIOUS EDITIONThis page from the 4th edition of Haematolymphoid Tumours is being updated. See 5th edition Table of Contents.
| This page is under construction |
Primary Author(s)*
Jay Alden, DO
Cancer Category/Type
Acute Myeloid Leukemia/Myeloid/lymphoid neoplasms
Cancer Sub-Classification / Subtype
Myeloid/lymphoid neoplasms with eosinophilia and gene rearrangement
Definition / Description of Disease
Myeloproliferative neoplasms associated with PDGFRA rearrangements are primary/neoplastic hypereosinophilic syndromes associated with recurrent rearrangements of the PDGFRA gene [1]. It is most commonly associated with FIP1L1-PDGFRA (F/P) fusion resulting from a cryptic deletion at 4q12, [2] and often presents as chronic eosinophilic leukemia (CEL), or less commonly, acute myeloid leukemia or T-lymphoblastic leukemia/lymphoma. [3]
Synonyms / Terminology
Chronic eosinophilic leukemia with FIP1L1-PDGFRA
FIP1L1-PDGFRA –associated chronic eosinophilic leukemia
Myeloid and lymphoid neoplasms associated with PDGFRA rearrangement
PDGFRA-associated Hypereosinophilic syndrome
Myeloid and lymphoid neoplasms with PDGFRA rearrangement
Myeloproliferative variant of the hypereosinophilic syndrome [4]
Epidemiology / Prevalence
The incidence and prevalence of myeloid/lymphoid neoplasms with PDGFRA rearrangement is not well characterized as demographic data is scarce [5]. The incidence of HES of any cause is estimated at 0.036 per 100,000, [6] though cases in which a causative genetic abnormality constitute a minority of these cases [5]. The F/P rearrangement is the most common abnormality identified, and is estimated to comprise approximately 10% of patients with significant hypereosinophilia [7] [8]. The entity is recently described, and disorders once called idiopathic hypereosinophilic syndrome are now being classified with genetic testing as specific primary neoplasms or reactive conditions. The F/P rearrangment is significantly more common in males with a male:female ratio of about 17:1. The age range varies from ages 7-77 with most patients being between 25 and 55 years. [9]
Clinical Features
Clinical presentation ranges from asymptomatic to fulminant, life threatening multi-system organ failure. Presenting signs and symptoms are typically related to eosinophilic infiltration, and consistent with hypereosinophilic syndromes of any cause. The largest clinical analysis of patients with hypereosinophilic syndromes (HES) demonstrated the following manifestations at presentation:[10]
- Dermatologic (eg, rash) – 57 percent
- Pulmonary (cough and breathlessness) – 25 percent
- Gastrointestinal – 14 percent
- Cardiac – <5 percent
- Asymptomatic -- 6 percent
Neoplastic PDGFRA-associated hypereosinophilic syndromes are more likely to present with eosinophilic cardiopulmonary disease than HES of any cause. A survey of 44 cases demonstrated skin, spleen, lung, and heart involvement in 57, 52, 45, and 34 percent of cases respectively with a similar rate of asymptomatic cases. [11]
Sites of Involvement
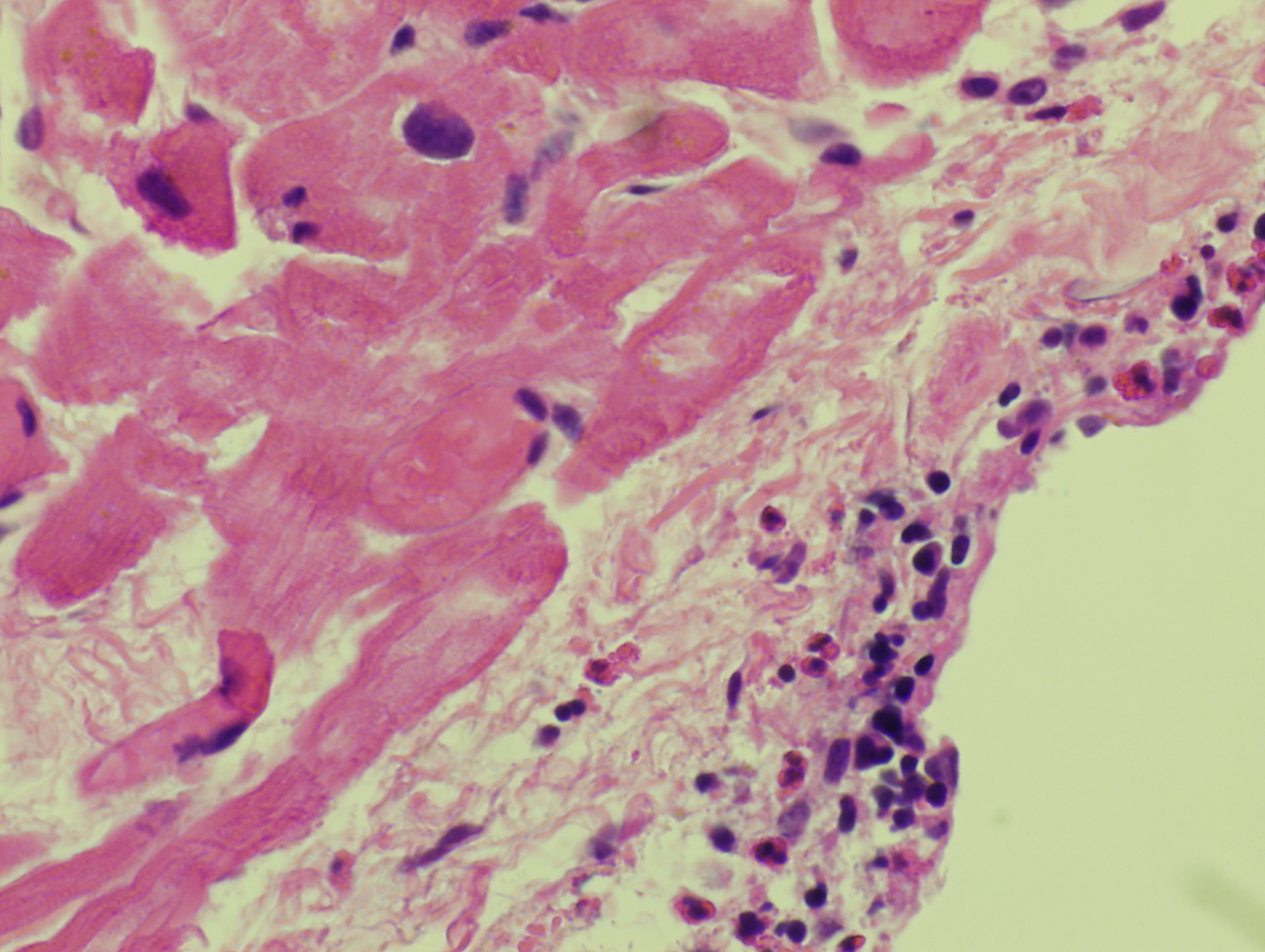
Leukemia associated with F/P is a systemic disease occupying the bone marrow and peripheral blood. Neoplastic cells may infiltrate various tissues such as the heart, lungs, nervous systems, skin and GI tract resulting in degranulation and cytokine mediated injury. [1]
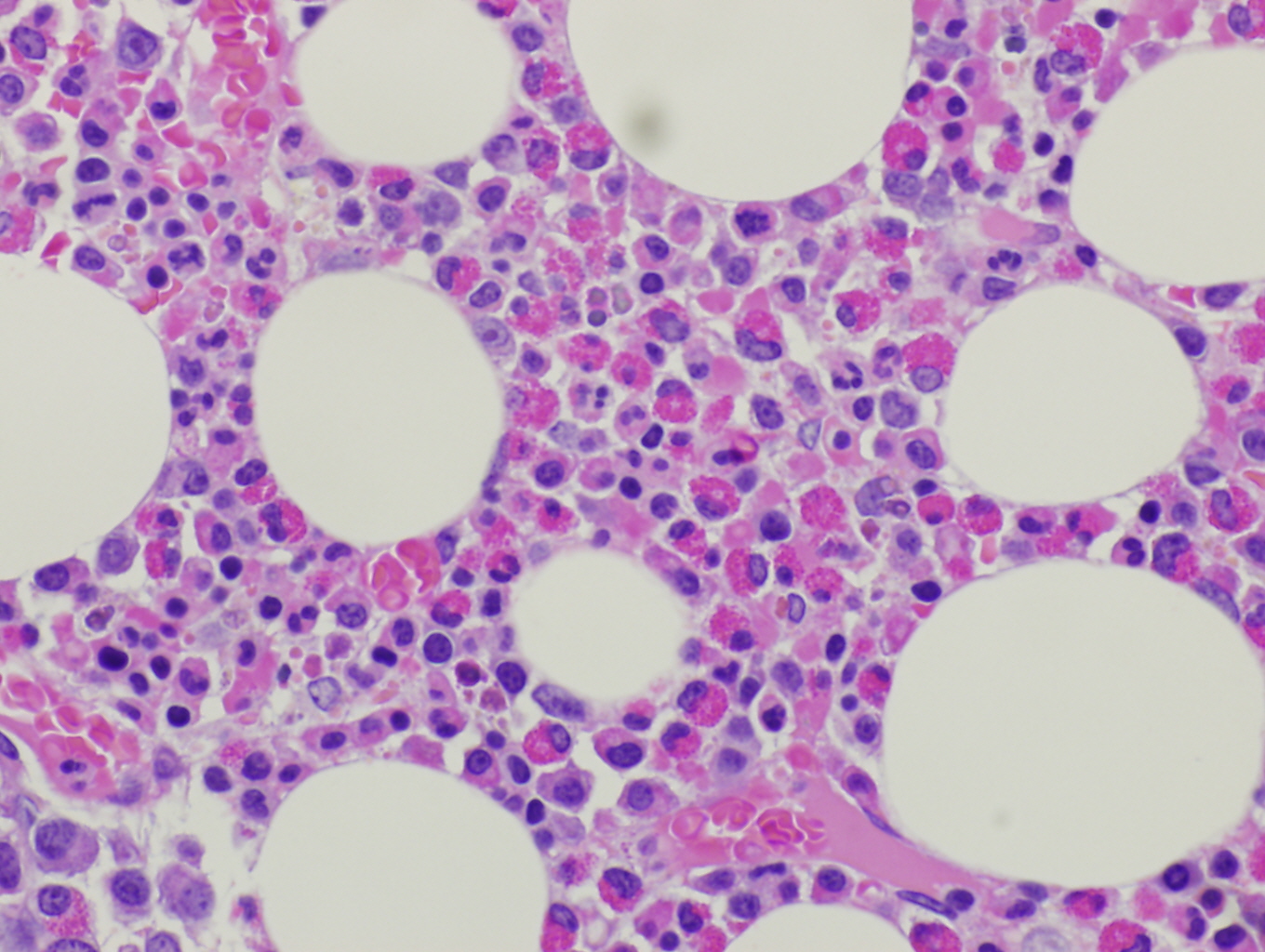
Morphologic Features
Histopathologic features are dependent on the organs involved. Eosinophilic infiltration is noted on diagnostic tissue biopsy. Eosinophilia and increased eosinophilic precursors can be identified on trephine bone marrow biopsy and peripheral blood smears. There is no histologic correlate to clonality, and genetic studies are required for diagnosis.
Immunophenotype
These neoplastic eosinophils may express markers of activation such as CD23, CD25, and CD69[4]. The basophils can sometimes be distinguished from those in systemic mastocytosis as CD2 is typically negative in the mast cells of PDGFRA rearrangement, but positive in systemic mastocytosis. [12][3]
| Finding | Marker |
|---|---|
| Positive (subset) | CD25 [4] |
| Positive (subset) | CD23 [4] |
| Positive (subset) | CD69 [4] |
| Negative (Mast cells) | CD2 [12][3] |
| Posivite (Mast cells, subset | CD25 [12][3] |
Chromosomal Rearrangements (Gene Fusions)
| Chromosomal Rearrangement | Genes in Fusion (5’ or 3’ Segments) | Prevalence |
|---|---|---|
| Cryptic del(4)(q12) | FIP1L1-PDGFRA | Majority |
| T(1;4) (q44;q12) | FIP1L1-PDGFRA | Rare [13] |
| T(4;10)(q12;p11.1-p11.2) | FIP1L1-PDGFRA | Rare [14] |
| T(4;22)(q12;q11.2) | BCR-PDGFRA | At least 9 cases [15][16][17][18] |
| T(2;4)(p24;q12) | STRN-PDGFRA | At least 1 case [19] |
| T(4;12)(q12;p13.2) | ETV6-PDGFRA | At least 1 case [19] |
| ins(9;4)(q33;q12q25) | CDK5RAP2-PDGFRA | At least 1 case [20] |
| T(4;10)(q12;q23.3) | TNKS2-PDGFRA | At least 1 case [21] |
Characteristic Chromosomal Aberrations / Patterns
Cytogenetic studies are usually normal though trisomy of chromosome 8 has been described, and may represent disease evolution [1].
Gene Mutations (SNV/INDEL)
An activating point mutation in PDGFRA has also been described [22].
Genes and Main Pathways Involved
the F/P tyrosine kinase is thought to become constitutively active in the setting of PDGRA juxtamembrane interruption as breakpoints in the PDGRA gene are tightly clustered, resulting in the removal of a portion of the juxtamembrane domain and activation of the kinase domain upon rearrangement. The role of the FIP1L1 in the neoplastic process is thought to be less significant. [23] The eosinophilic proliferation observed in these patients is thought to result from multiple signalling pathways including phosphoinositol 3-kinase, ERK 1/2 and STAT5, though the precise mechanism remains elusive. [2][24]
Diagnostic Testing Methods
Myeloid/lymphoid neoplasms with PDGFRA are diagnosed with a combination of morphologic, immunophenotypic and genomic modalities; typically with a bone marrow biopsy and peripheral blood smear review, preferably drawn prior to administration of high dose corticosteroids. Additional studies performed at the time of workup include CBC to quantify any abnormalities in other cell lines, tests of hepatic and renal function, troponin for evidence of myocarditis, vitamin B12, serum tryptase, antineutrophil cytoplasmic antibodies, and a high resolution chest CT for evidence of pulmonary involvement. [25] The diagnosis is made when the F/P fusion gene or a variant fusion gene with rearrangement of PDGFRA or an activating mutation of PDGFRA is identified in the setting of a myeloid or lymphoid neoplasm, usually with prominent eosinophilia [1].
The F/P fusion gene can be detected by reverse transcriptase PCR (RT-PCR) [13], or the deletion can be detected using a probe for the CHIC2 gene, or with a break apart probe encompassing FIP1L1 and PDGFRA. [1]
Clinical Significance (Diagnosis, Prognosis and Therapeutic Implications)
The responsiveness of F/P associated myeloid/lymphoid neoplasms to imatinib mesylate is well documented [5]. Adverse outcomes are typically related to late presentation, where irreversible organ damage precedes diagnosis, or when the disease is diagnosed in an accelerated phase when complications are more likely. Induction dosing of imatinib ranges from 100-400 mg daily, with much lower maintenence dosing recommended to prevent relapse [26] [27]. Complete hematologic and molecular remission is observed in nearly all patients taking imatinib, usually within 3 months. [28] [29] [30] Imatinib maintains efficacy in accelerated or blast phase disease, and resistance is rare [11] [31].
Familial Forms
Put your text here
Other Information
Put your text here
Links
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 Bain BJ, et al., (2017). Myeloid/lymphoid neoplasms with PDGFRA rearrangement in World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues, Revised 4th edition. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Arber DA, Hasserjian RP, Le Beau MM, Orazi A, and Siebert R, Editors. IARC Press: Lyon, France, p73-75.
- ↑ Jump up to: 2.0 2.1 Cools, Jan; et al. (2003). "A Tyrosine Kinase Created by Fusion of the PDGFRA and FIP1L1 Genes as a Therapeutic Target of Imatinib in Idiopathic Hypereosinophilic Syndrome". New England Journal of Medicine. 348 (13): 1201–1214. doi:10.1056/NEJMoa025217. ISSN 0028-4793.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 Metzgeroth, G.; et al. (2007). "Recurrent finding of the FIP1L1-PDGFRA fusion gene in eosinophilia-associated acute myeloid leukemia and lymphoblastic T-cell lymphoma". Leukemia. 21 (6): 1183–1188. doi:10.1038/sj.leu.2404662. ISSN 0887-6924. PMID 17377585.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 4.4 Klion, Amy D.; et al. (2004). "Molecular remission and reversal of myelofibrosis in response to imatinib mesylate treatment in patients with the myeloproliferative variant of hypereosinophilic syndrome". Blood. 103 (2): 473–478. doi:10.1182/blood-2003-08-2798. ISSN 0006-4971. PMID 14504092.
- ↑ Jump up to: 5.0 5.1 5.2 Shomali, William; et al. (2019). "World Health Organization-defined eosinophilic disorders: 2019 update on diagnosis, risk stratification, and management". American Journal of Hematology. 94 (10): 1149–1167. doi:10.1002/ajh.25617. ISSN 1096-8652. PMID 31423623.
- ↑ Crane, Martin M.; et al. (2010). "Incidence of myeloproliferative hypereosinophilic syndrome in the United States and an estimate of all hypereosinophilic syndrome incidence". The Journal of Allergy and Clinical Immunology. 126 (1): 179–181. doi:10.1016/j.jaci.2010.03.035. ISSN 1097-6825. PMC 5781228. PMID 20639012.
- ↑ Pardanani, Animesh; et al. (2004). "FIP1L1-PDGFRA fusion: prevalence and clinicopathologic correlates in 89 consecutive patients with moderate to severe eosinophilia". Blood. 104 (10): 3038–3045. doi:10.1182/blood-2004-03-0787. ISSN 0006-4971. PMID 15284118.
- ↑ Pardanani, A.; et al. (2006). "FIP1L1-PDGFRA in eosinophilic disorders: prevalence in routine clinical practice, long-term experience with imatinib therapy, and a critical review of the literature". Leukemia Research. 30 (8): 965–970. doi:10.1016/j.leukres.2005.11.011. ISSN 0145-2126. PMID 16406016.
- ↑ Bain, Barbara J.; et al. (2007). "Chronic eosinophilic leukemias and the myeloproliferative variant of the hypereosinophilic syndrome". Immunology and Allergy Clinics of North America. 27 (3): 377–388. doi:10.1016/j.iac.2007.06.001. ISSN 0889-8561. PMID 17868855.
- ↑ Ogbogu, Princess U.; et al. (2009). "Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy". The Journal of Allergy and Clinical Immunology. 124 (6): 1319–1325.e3. doi:10.1016/j.jaci.2009.09.022. ISSN 1097-6825. PMC 2829669. PMID 19910029.
- ↑ Jump up to: 11.0 11.1 Legrand, Fanny; et al. (2013). "The Spectrum of FIP1L1-PDGFRA-Associated Chronic Eosinophilic Leukemia: New Insights Based on a Survey of 44 Cases". Medicine. 92 (5): e1–e9. doi:10.1097/MD.0b013e3182a71eba. ISSN 1536-5964. PMC 4553979. PMID 23982058.
- ↑ Jump up to: 12.0 12.1 12.2 Klion, Amy D.; et al. (2003). "Elevated serum tryptase levels identify a subset of patients with a myeloproliferative variant of idiopathic hypereosinophilic syndrome associated with tissue fibrosis, poor prognosis, and imatinib responsiveness". Blood. 101 (12): 4660–4666. doi:10.1182/blood-2003-01-0006. ISSN 0006-4971. PMID 12676775.
- ↑ Jump up to: 13.0 13.1 Cools, Jan; et al. (2003). "A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome". The New England Journal of Medicine. 348 (13): 1201–1214. doi:10.1056/NEJMoa025217. ISSN 1533-4406. PMID 12660384.
- ↑ Tashiro, Haruko; et al. (2006). "Molecular analysis of chronic eosinophilic leukemia with t(4;10) showing good response to imatinib mesylate". International Journal of Hematology. 83 (5): 433–438. doi:10.1532/IJH97.05180. ISSN 0925-5710. PMID 16787876.
- ↑ Baxter, E. Joanna; et al. (2002). "The t(4;22)(q12;q11) in atypical chronic myeloid leukaemia fuses BCR to PDGFRA". Human Molecular Genetics. 11 (12): 1391–1397. doi:10.1093/hmg/11.12.1391. ISSN 0964-6906. PMID 12023981.
- ↑ Van Etten, Richard A.; et al. (2006). "Distinct Leukemogenic Activity and Imatinib Responsiveness of a BCR-PFGFRα Fusion Tyrosine Kinase". Blood. 108 (11): 3634–3634. doi:10.1182/blood.V108.11.3634.3634. ISSN 0006-4971.
- ↑ Safley, Anne Michele; et al. (2004). "Molecular and cytogenetic characterization of a novel translocation t(4;22) involving the breakpoint cluster region and platelet-derived growth factor receptor-alpha genes in a patient with atypical chronic myeloid leukemia". Genes, Chromosomes & Cancer. 40 (1): 44–50. doi:10.1002/gcc.20014. ISSN 1045-2257. PMID 15034867.
- ↑ Trempat, Pascal; et al. (2003). "Chronic myeloproliferative disorders with rearrangement of the platelet-derived growth factor alpha receptor: a new clinical target for STI571/Glivec". Oncogene. 22 (36): 5702–5706. doi:10.1038/sj.onc.1206543. ISSN 0950-9232. PMID 12944919.
- ↑ Jump up to: 19.0 19.1 Curtis, Claire E.; et al. (2007). "Two novel imatinib-responsive PDGFRA fusion genes in chronic eosinophilic leukaemia". British Journal of Haematology. 138 (1): 77–81. doi:10.1111/j.1365-2141.2007.06628.x. ISSN 0007-1048. PMID 17555450.
- ↑ Walz, Christoph; et al. (2006). "Transient response to imatinib in a chronic eosinophilic leukemia associated with ins(9;4)(q33;q12q25) and a CDK5RAP2-PDGFRA fusion gene". Genes, Chromosomes & Cancer. 45 (10): 950–956. doi:10.1002/gcc.20359. ISSN 1045-2257. PMID 16845659.
- ↑ Chalmers, Z. R.; et al. (2015). "Comprehensive genomic profiling identifies a novel TNKS2-PDGFRA fusion that defines a myeloid neoplasm with eosinophilia that responded dramatically to imatinib therapy". Blood Cancer Journal. 5: e278. doi:10.1038/bcj.2014.95. ISSN 2044-5385. PMC 4349257. PMID 25658984.
- ↑ Elling, Christian; et al. (2011). "Novel imatinib-sensitive PDGFRA-activating point mutations in hypereosinophilic syndrome induce growth factor independence and leukemia-like disease". Blood. 117 (10): 2935–2943. doi:10.1182/blood-2010-05-286757. ISSN 1528-0020. PMID 21224473.
- ↑ J. Cools; et al. (2008). "Five years since the discovery of FIP1L1–PDGFRA : what we have learned about the fusion and other molecularly defined eosinophilias". Leukemia. 22 (11): 1999–2010. doi:10.1038/leu.2008.287. ISSN 1476-5551.
- ↑ Buitenhuis, Miranda; et al. (2007). "Molecular mechanisms underlying FIP1L1-PDGFRA-mediated myeloproliferation". Cancer Research. 67 (8): 3759–3766. doi:10.1158/0008-5472.CAN-06-4183. ISSN 0008-5472. PMID 17440089.
- ↑ Rosemarin, A, and Feldweg, A. Hypereosinophilic syndromes: Treatement. In: UpToDate, Post, TW (Ed), UpToDate, Waltham, MA. April 2020
- ↑ Baccarani, Michele; et al. (2007). "The efficacy of imatinib mesylate in patients with FIP1L1-PDGFRalpha-positive hypereosinophilic syndrome. Results of a multicenter prospective study". Haematologica. 92 (9): 1173–1179. doi:10.3324/haematol.11420. ISSN 1592-8721. PMID 17666373.
- ↑ Jovanovic, Jelena V.; et al. (2007). "Low-dose imatinib mesylate leads to rapid induction of major molecular responses and achievement of complete molecular remission in FIP1L1-PDGFRA-positive chronic eosinophilic leukemia". Blood. 109 (11): 4635–4640. doi:10.1182/blood-2006-10-050054. ISSN 0006-4971. PMID 17299092.
- ↑ Baccarani, Michele; et al. (2007). "The efficacy of imatinib mesylate in patients with FIP1L1-PDGFRalpha-positive hypereosinophilic syndrome. Results of a multicenter prospective study". Haematologica. 92 (9): 1173–1179. doi:10.3324/haematol.11420. ISSN 1592-8721. PMID 17666373.
- ↑ Quéméneur, Thomas; et al. (2013). "Systemic vasculitis during the course of systemic sclerosis: report of 12 cases and review of the literature". Medicine. 92 (1): 1–9. doi:10.1097/MD.0b013e31827781fd. ISSN 1536-5964. PMC 5370746. PMID 23263715.
- ↑ Helbig, Grzegorz; et al. (2008). "A single weekly dose of imatinib is sufficient to induce and maintain remission of chronic eosinophilic leukaemia in FIP1L1-PDGFRA-expressing patients". British Journal of Haematology. 141 (2): 200–204. doi:10.1111/j.1365-2141.2008.07033.x. ISSN 1365-2141. PMID 18307562.
- ↑ Lierman, E.; et al. (2009). "FIP1L1-PDGFRalpha D842V, a novel panresistant mutant, emerging after treatment of FIP1L1-PDGFRalpha T674I eosinophilic leukemia with single agent sorafenib". Leukemia. 23 (5): 845–851. doi:10.1038/leu.2009.2. ISSN 1476-5551. PMID 19212337.
Notes
*Primary authors will typically be those that initially create and complete the content of a page. If a subsequent user modifies the content and feels the effort put forth is of high enough significance to warrant listing in the authorship section, please contact the CCGA coordinators (contact information provided on the homepage). Additional global feedback or concerns are also welcome.