HAEM4Backup:Waldenstrom Macroglobulinemia
Primary Author(s)*
Kapitolina Semenova, MD, Jack Reid, MD , Fabiola Quintero-Rivera, MD*
Departments of Pathology, Laboratory Medicine, and *Pediatrics, Division of Genetic and Genomic Medicine, University of California, Irvine (UCI)
Cancer Category/Type
Non-Hodgkin lymphoma.
Cancer Sub-Classification / Subtype
Low-grade B-cell clonal neoplasm.
Definition / Description of Disease
Waldenström macroglobulinemia (WM) is a clinicopathological entity characterized by presence of Lymphoplasmacytic lymphoma (LPL) associated with any level of monoclonal immunoglobulin M (IgM) in the serum.
Synonyms / Terminology
Waldenström's macroglobulinemia, Waldenstrom macroglobulinemia, Macroglobulinemia.
Epidemiology / Prevalence
- Waldenström macroglobulinemia is a rare disorder with an age-adjusted incidence rate of 3.4 among the male population and 1.7 in the female population. [1]
- WM occurs most frequently in Caucasians and is less common in Asian and African-American populations. [2]
- WM is more common in men, with male to female ratio of approximately 2:1. [3]
- The median age of 60-70 years. [2]
- Approximately 2% of all hematologic malignancies with 1000-1500 new cases per year in the United States are represented by WM. [4]
Clinical Features
- WM is an indolent disease with a wide variety of symptoms which could be divided in two categories: neoplastic organ involvement and IgM paraprotein-related symptoms. [4]
- Approximately 30% of patients are asymptomatic. [2][3]
- Symptomatic hyperviscosity is observed in patients with IgM levels >30 g/l. [5]
- Approximately 3% of patients have systemic amyloidosis, more often of immunoglobulin light chain (AL) type. [6]
| Signs and Symptoms | B-symptoms (fever, weight loss, fatigue, night sweats)
Bleeding Lymphadenopathy Hepatosplenomegaly IgM related symptoms:
|
| Laboratory Findings | Anemia, usually mild normochromic and normocytic[9]Thrombocytopenia
Elevated beta2-microglobulin (increased in approximately 50% of patients)[3] High erythrocyte sedimentation rate Hypoalbuminemia (<30.0 g/L)[10] High serum IgM Cold agglutinin hemolytic anemia (rare, observed in 1.5% of patients)[3] Cryoglobulinemia (associated with concurrent hepatitis C infection) Monoclonal immunoglobulin light chains (Bence-Jones proteins) in the urine in up to 80% of patients with WM.[9] |
Sites of Involvement
- By definition, diagnosis of Waldenström macroglobulinemia requires bone marrow involvement by Lymphoplasmacytic lymphoma.
- Lymphadenopathy and hepatosplenomegaly are more common at the time of relapse (up to 50%) rather than at the time of initial presentation (20%). [11]
- Peripheral blood may have lymphocytosis with small circulating neoplastic cells with condensed chromatin and inconspicuous nucleoli. Rouleaux formation is also observed. [9]
- Extramedullary involvement has been reported in <5% of patients with WM, with the lungs, soft tissue, central nervous system, kidneys, and bones being the most common sites. [12]
- The pulmonary system can be involved by WM in the form of malignant pleural effusion[13] as well as an infiltration of the parenchyma of the lung. [12]
- Soft tissue involvement is reported in 21% of patients with an extramedullary presentation of WM and appears as a soft tissue mass in various locations. [12]
- Rare central nervous system involvement by WM lymphoplasmacytic infiltrate is known as Bing-Neel syndrome. [14] Bing -Neel syndrome is characterized by direct infiltration of the central nervous system and clinical symptoms such as headache, vertigo, ataxia, diplopia, impaired hearing. [12]
- Kidney involvement may present as nephrotic syndrome and demonstrate renal localization of the lymphoplasmacytic infiltrate of the WM. [15][16]
- Bone involvement is reported in 9% of patients with an extramedullary presentation of WM. [12]
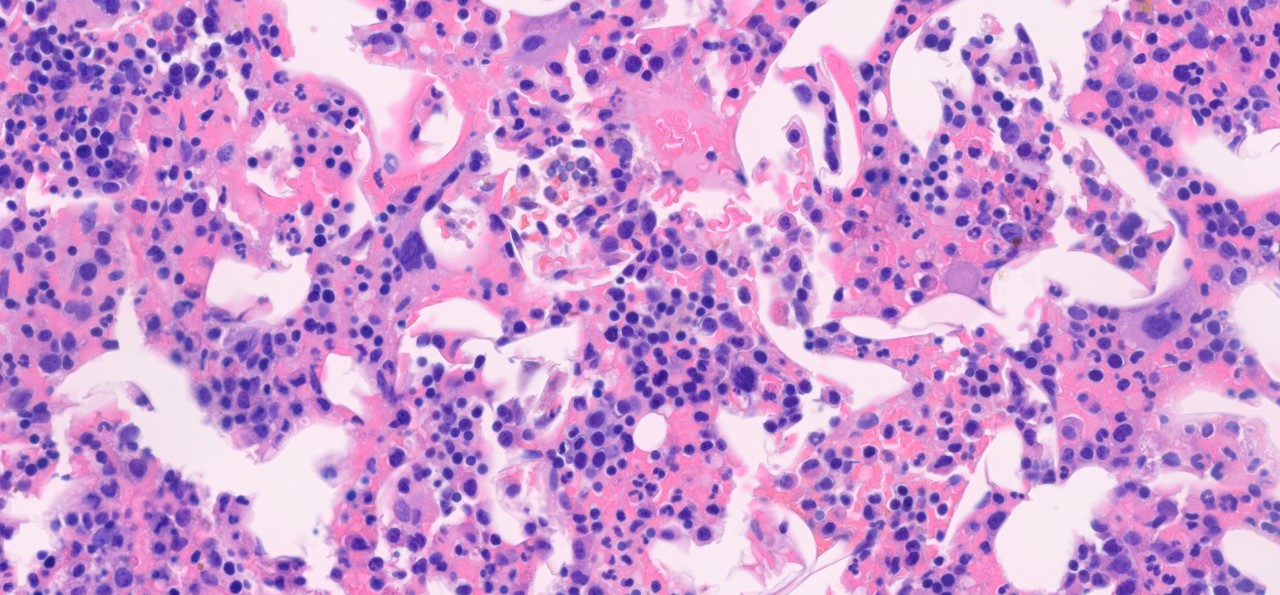
Morphologic Features
- Bone marrow involvement by lymphoplasmacytic lymphoma is characterized by an interstitial, diffuse, or nodular patterns of infiltration. [2]
- Tumor burden of the bone marrow is variable with a wide range of percentage of involvement (5-95%). [10]
- Paratrabecular infiltration is composed of nodular aggregates or single cells along the bone trabeculae with peritrabecular fibrosis and is seen in 95% of cases. [17]
- Lymphoplasmacytic infiltrate is composed mainly of small monotonous lymphocytes with a various numbers of plasma cells and plasmacytoid lymphocytes. [2]
- An increased number of reactive mast cells in the background stroma of lymphoid infiltrates is often present. [17]
- Dutcher bodies (intracytoplasmic immunoglobulin inclusions) and Russell bodies (mucopolysaccharides and immunoglobulin inclusion in the rough endoplasmic reticulum) are often present within plasma cells. [2][18]
Immunophenotype[2]
| Finding | Marker |
|---|---|
| Positive (universal) | IgM |
| Positive (universal) | CD19 |
| Positive (universal) | CD20 |
| Positive (universal) | CD22 (dim) |
| Positive (universal) | CD25 |
| Positive (universal) | CD79a |
| Positive (universal) | CD45 |
| Positive (subset) | CD38 |
| Positive (subset) | CD138 |
| Positive (universal) | FMC7 |
| Negative (universal) | CD5 |
| Negative (subset) | CD10 |
| Negative (universal) | CD56 |
| Negative (universal) | CD117 |
| Negative (universal) | LEF1 |
| Negative (universal) | Cyclin D1 |
| Negative (universal) | EBV |
- Lymphocytes express B cell antigens and are positive for CD19, CD20, CD79a, and CD22. Plasma cells express CD138 and CD38.
- The majority of lymphocytes express function-associated antigen (LFA-1). [19]
- Adhesion molecules such as L-selectin, ICAM-1, CD44, and CD11c are expressed in a subset of cases of WM.
- Up to 20% may express CD5, CD10, and CD23. [20]
Chromosomal Rearrangements (Gene Fusions)
- t(9;14)(p13;q32) has been initially identified in the extensive retrospective analysis and correlated with a plasmacytoid differentiation. [21] This translocation has not been observed in a newer study. [10]
- Rare cases associated with t(8;14)(q24.1;q32), t(11;18)(q21;q21), and t(14;18)(q32;q21) were observed in rare case reports. [22][23][24]
Individual Region Genomic Gain/Loss/LOH
- Chromosome 6q deletion involving q13-q22 regions is the most frequent structural abnormality.
- Deletion 6q is associated with adverse prognostic features. This deletion can be found in other B-cell neoplasm.
- The presence of 6q deletion is suggested as a marker of transformation to large cell lymphomas. Monosomy of chromosome 8 has also been reported in a few cases[10] This region includes genes of several modulators such as NF-kappa-B, BCL2, apoptosis, and plasma cell differentiation, which can participate in the pathogenesis of the WM/LPL.[19]
| Chr # | Gain / Loss / Amp / LOH | Minimal Region Genomic Coordinates [Genome Build] | Minimal Region Cytoband | Diagnostic Significance (Yes, No or Unknown) | Prognostic Significance (Yes, No or Unknown) | Therapeutic Significance (Yes, No or Unknown) | Notes |
|---|---|---|---|---|---|---|---|
| 3 | Gain | Trisomy 3; 3q21-23 to 3q25-29 are in the minimal overrepresented region | No | No | No | Trisomy 3 is reported in rare case series.[10] | |
| 4 | Gain | Trisomy 4 | Yes | Yes | No | Trisomy 4 is associated with adverse clinical presentation.[25] | |
| 5 | Gain | Trisomy 5 | No | No | No | Trisomy 5 is reported in rare case series.[10] | |
| 6 | Loss | Del(6q) | No | No | No | Deletion of chromosome 6q is the most frequent chromosomal abnormality reported in up to 60% in patients with WM.[2] Deletion 6q has no prognostic importance; however it has been reported in association with features of adverse prognosis.[25] | |
| 11 | Loss | Del(11q) | No | No | No | ||
| 12 | Gain | Trisomy 12 | No | No | No | Rare (<5%); Trisomy 12 associated with short progression-free survival.[25] | |
| 18 | Gain | Trisomy 18 | No | No | No | Common secondary finding in B-cell neoplams. | |
| 17 | Loss | Del(17p) | No | No | No | 17p (T53) deletion is found in 8% of cases.[25] |
Characteristic Chromosomal Patterns
- There are no characteristic chromosomal patterns in WM.
- Trisomy of chromosome 4 and trisomy of chromosome 18 was shown in association. [25]
Gene Mutations (SNV/INDEL)[26]
- In symptomatic patients with WM, three or more copy number alterations (CNA) were reported with higher frequency than in asymptomatic patients.
| Gene; Genetic Alteration | Presumed Mechanism (Tumor Suppressor Gene [TSG] / Oncogene / Other) | Prevalence (COSMIC / TCGA / Other) | Concomitant Mutations | Mutually Exclusive Mutations | Diagnostic Significance (Yes, No or Unknown) | Prognostic Significance (Yes, No or Unknown) | Therapeutic Significance (Yes, No or Unknown) | Notes |
|---|---|---|---|---|---|---|---|---|
| TNFAIP3 gene; 6q MDRs | TSG | 90% of patients have concomitant aberrations such as gain of chromosome 4, gain of 6p, or deletion of 13q. | Unknown | Unknown | Unknown | Unknown | ||
| Potential involved genes CASP3, SETS7, CAMK2D; MAR1 (4q24), MAR2 (4q31-4q35) | Unknown | Unknown | Unknown | Unknown | Unknown | ATM gene plays in DNA damage response pathway. | ||
| ATM; deletion 11q22.3-q23 | Unknown | Unknown | Unknown | Unknown | Unknown | |||
| DLEU7; methylation | Unknown | Unknown | Unknown | Unknown | Unknown | DLEU7 is a regulator of NF-kB pathway. | ||
| TP53; 17p13 deletion | TSG | Unknown | Unknown | Unknown | Unknown | Unknown | ||
| CD79B/CD79A | Unknown | Unknown | Unknown | Unknown | Unknown | Key component of BCR pathway; Mutations in CD79B/CD79A were observed in 15% of patients. No correlation with clinical data was identified. |
Note: A more extensive list of mutations can be found in cBioportal (https://www.cbioportal.org/), COSMIC (https://cancer.sanger.ac.uk/cosmic), ICGC (https://dcc.icgc.org/) and/or other databases. When applicable, gene-specific pages within the CCGA site directly link to pertinent external content.
Epigenomic Alterations
MicroRNA-155 as well as other microRNAs (miRNA-184, -206, -363, -494, -542-3p) are upregulated in the cells derived from the bone marrow of patients with WM.[27] Recent studies have demonstrated that miRNA act as a modulator of histone acetylation and therefore may be considered as an epigenetic alteration.[27][28]
MicroRNA-155 loss of function were shown to correlate with:
- Decline in WM cell proliferation
- Downregulation of PI3K/AKT and MAPK/ERK signaling pathways
- Inhibition of NFkB activation
- Inhibition of WM cells adhesion to fibronectin
- Inhibition of WM cell migration to SDF-1[27]
Partial methylation of DLEU7 gene, which regulates NF-kB pathway, was seen in all patients regardless of clinical status.[26]
Genes and Main Pathways Involved
- MYD88 L265, a single point mutation at NM_002468:c.978T>C (rs387907272) resulting in a p.Leu265Pro (L265P) amino acid change is the most common somatic mutation in patients with WM reported in more than 90% of patients. [4][29][30] MYD88 is an adaptor protein for toll-like receptor 4 (TLR-4) and also for interleukin-1 and -2 receptors (IL-1R and IL-2R).
- MYD88 L265P mutation may not be enough to cause malignant transformation. It may represent a part of a preneoplastic landscape that, together with other genetic alterations, causes the progression to the disease. [31]
- CXCR4 C1013G is the second most common somatic mutation involving the C-terminal domain of CXCR4 identified in patients with Waldenström macroglobulinemia and lymphoplasmacytic lymphoma and presents in up to 40% of patients with WM. Somatic mutations almost always occur in those with MYD88 mutations, some patients with wild-type MYD88 can also express CXCR4 mutations[29] .
- CXCR4 mutations are essentially unique to WM, with only a few cases of marginal zone lymphoma and activated B-cell (ABC) subtype of DLBCL reported so far. Germline mutations in the C-terminal domain of CXCR4 are present in patients with WHIM (autosomal dominant warts, hypogammaglobulinemia, infection, and myelokathexis) syndrome[29] The C-X-C chemokine receptor type 4 (CXCR4) is a G-protein-coupled receptor that regulates cell trafficking in hematopoietic stem cells and clonal B cells. [32]
- The presence of CXCR4 mutations is associated with a higher bone marrow burden. Patients with nonsense CXCR4 mutation was reported in association with higher immunoglobulin M level and symptomatic hyperviscosity. [33]
- In cases with mutated ARID1A WM, CXCL13 was shown to be overexpressed and correlated with bone marrow involvement. [32]
- Recurrent somatic mutations in CD79B (8-15%) [29], KMPTD/MLL2 (mutated in 7%), TP53 (mutated in 7%), MYBBP1A (mutated in 7%) [20] are also reported.
- Patients with wild-type MYD88 WM were shown to have somatic mutations such as TBL1XR1, PTPN13, MALT1, BCL10, NFKB2, NFKBIB, NFKBIZ, and UDRL1F associated with activation of NF-kB and other mutations associated with epigenomic dysregulation (KMT2D, KMT2C, and KDM6A), and mutations causing DNA damage repair (TP53, ATM, TRRAP). [34]
- Patients with MYD88 wild-type WM show worse prognosis compare to patients with mutated MYD88 WM. Also, the BTK inhibitor ibrutinib is less effective in patients with wild-type MYD88 WM. [34]
| Gene; Genetic Alteration | Pathway | Pathophysiologic Outcome |
|---|---|---|
| MYD88 L265; Activating mutation | The transcription factor nuclear factor (NF)-kB pathway | Enhanced cell survival[35] |
| CXCR4 C1013G; Activating mutation | Cytokine release and chemotaxis | WM cells homing; promote WM cells dissemination[36] |
| ARID1A | Part of switch/sucrose nonfermentable family; Chromatin remodeling protein | Poorly understood, however some studies suggest a possible role in modulating TP53 gene with an epigenetic tumor suppressor function.[33]Patients with ARID1A mutated WM show higher bone marrow disease compare to non-ARID1A mutated WM patients.[32] |
Genetic Diagnostic Testing Methods
- Next-generation sequencing is used to detect recurrent somatic mutations such as MYD88, CXCR4, ARID1A, and CD79B.
- Allele-specific polymerase chain reaction (AS-PCR) can be used to detect MYD88 L265P mutated cells in the peripheral blood. [29]
Familial Forms
- The majority of cases of WM are sporadic; however, approximately 20% of cases have been shown in association with WM in first-degree relatives, which may suggest an autosomal dominant or co-dominant mode of inheritance. [37]
- Kristinsson et al. showed a 20-fold increased risk of developing Lymphoplasmacytic lymphoma or WM in first-degree relatives with WM and an increased risk of acquiring other hematologic malignancies. [38]
- Certain germline variants and BCL2 overexpression have been suggested to be predisposing factors; however, further investigation is required. [39]
Additional Information
Put your text here
Links
Put your text placeholder here (use "Link" icon at top of page)
References
- ↑ Groves, F. D.; et al. (1998-03-15). "Waldenström's macroglobulinemia: incidence patterns in the United States, 1988-1994". Cancer. 82 (6): 1078–1081. ISSN 0008-543X. PMID 9506352.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Wang, Wei; et al. (2020-01). "Lymphoplasmacytic lymphoma and Waldenström macroglobulinaemia: clinicopathological features and differential diagnosis". Pathology. 52 (1): 6–14. doi:10.1016/j.pathol.2019.09.009. ISSN 1465-3931. PMID 31767130. Check date values in:
|date=(help) - ↑ 3.0 3.1 3.2 3.3 García-Sanz, R.; et al. (2001-12). "Waldenström macroglobulinaemia: presenting features and outcome in a series with 217 cases". British Journal of Haematology. 115 (3): 575–582. doi:10.1046/j.1365-2141.2001.03144.x. ISSN 0007-1048. PMID 11736938. Check date values in:
|date=(help) - ↑ 4.0 4.1 4.2 4.3 Yun, Seongseok; et al. (2017-05). "Waldenström Macroglobulinemia: Review of Pathogenesis and Management". Clinical Lymphoma, Myeloma & Leukemia. 17 (5): 252–262. doi:10.1016/j.clml.2017.02.028. ISSN 2152-2669. PMC 5413391. PMID 28366781. Check date values in:
|date=(help) - ↑ Gustine, Joshua N.; et al. (2017-06). "Serum IgM level as predictor of symptomatic hyperviscosity in patients with Waldenström macroglobulinaemia". British Journal of Haematology. 177 (5): 717–725. doi:10.1111/bjh.14743. ISSN 1365-2141. PMID 28485115. Check date values in:
|date=(help) - ↑ Palladini, Giovanni; et al. (2013-04). "Diagnostic challenges of amyloidosis in Waldenström macroglobulinemia". Clinical Lymphoma, Myeloma & Leukemia. 13 (2): 244–246. doi:10.1016/j.clml.2013.02.001. ISSN 2152-2669. PMID 23474147. Check date values in:
|date=(help) - ↑ Menke, Marcel N.; et al. (2006-11). "Hyperviscosity-related retinopathy in waldenstrom macroglobulinemia". Archives of Ophthalmology (Chicago, Ill.: 1960). 124 (11): 1601–1606. doi:10.1001/archopht.124.11.1601. ISSN 0003-9950. PMID 17102008. Check date values in:
|date=(help) - ↑ Lipsker, Dan (2010-12-08). "The Schnitzler syndrome". Orphanet Journal of Rare Diseases. 5: 38. doi:10.1186/1750-1172-5-38. ISSN 1750-1172. PMC 3018454. PMID 21143856.
- ↑ 9.0 9.1 9.2 Naderi, Nadia; et al. (2013-04). "Lymphoplasmacytic lymphoma and Waldenström macroglobulinemia". Archives of Pathology & Laboratory Medicine. 137 (4): 580–585. doi:10.5858/arpa.2012-0034-RS. ISSN 1543-2165. PMID 23544948. Check date values in:
|date=(help) - ↑ 10.0 10.1 10.2 10.3 10.4 10.5 Mansoor, A.; et al. (2001-10). "Cytogenetic findings in lymphoplasmacytic lymphoma/Waldenström macroglobulinemia. Chromosomal abnormalities are associated with the polymorphous subtype and an aggressive clinical course". American Journal of Clinical Pathology. 116 (4): 543–549. doi:10.1309/6U88-357U-UKJ5-YPT3. ISSN 0002-9173. PMID 11601139. Check date values in:
|date=(help) - ↑ Shaheen, Saad P.; et al. (2012-01). "Waldenström macroglobulinemia: a review of the entity and its differential diagnosis". Advances in Anatomic Pathology. 19 (1): 11–27. doi:10.1097/PAP.0b013e31824019d0. ISSN 1533-4031. PMID 22156831. Check date values in:
|date=(help) - ↑ 12.0 12.1 12.2 12.3 12.4 Banwait, Ranjit; et al. (2015-02). "Extramedullary Waldenström macroglobulinemia". American Journal of Hematology. 90 (2): 100–104. doi:10.1002/ajh.23880. ISSN 1096-8652. PMID 25349134. Check date values in:
|date=(help) - ↑ Barnes, Martin; et al. (2020-07-13). "Pleural fluid MYD88 L265P mutation supporting diagnosis and decision to treat extramedullary Waldenstrom's macroglobulinemia: a case report". Journal of Medical Case Reports. 14 (1): 98. doi:10.1186/s13256-020-02404-x. ISSN 1752-1947. PMC 7358196 Check
|pmc=value (help). PMID 32654665 Check|pmid=value (help). - ↑ Arjunan, Ananth; et al. (2019). "Central Nervous System Involvement by Waldenstrom Macroglobulinemia: A Case Report of the Bing-Neel Syndrome". Case Reports in Hematology. 2019: 4075960. doi:10.1155/2019/4075960. ISSN 2090-6560. PMC 6437752. PMID 31001436.
- ↑ Chauvet, Sophie; et al. (2015-11). "Kidney diseases associated with monoclonal immunoglobulin M-secreting B-cell lymphoproliferative disorders: a case series of 35 patients". American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation. 66 (5): 756–767. doi:10.1053/j.ajkd.2015.03.035. ISSN 1523-6838. PMID 25987261. Check date values in:
|date=(help) - ↑ Salviani, Chiara; et al. (2014-02). "Renal involvement in Waldenström's macroglobulinemia: case report and review of literature". Renal Failure. 36 (1): 114–118. doi:10.3109/0886022X.2013.832859. ISSN 1525-6049. PMID 24059636. Check date values in:
|date=(help) - ↑ 17.0 17.1 Bassarova, Assia; et al. (2015-06). "Lymphoplasmacytic lymphoma and marginal zone lymphoma in the bone marrow: paratrabecular involvement as an important distinguishing feature". American Journal of Clinical Pathology. 143 (6): 797–806. doi:10.1309/AJCP6ZODWV1CIDME. ISSN 1943-7722. PMID 25972321. Check date values in:
|date=(help) - ↑ Han, Jae Ho; et al. (2019-01). "An old misconception: Dutcher bodies are intranuclear inclusions in plasma cells?". Leukemia & Lymphoma. 60 (1): 265–267. doi:10.1080/10428194.2018.1461864. ISSN 1029-2403. PMID 29846124. Check date values in:
|date=(help) - ↑ 19.0 19.1 "UpToDate".
- ↑ 20.0 20.1 Hunter, Zachary R.; et al. (2005-03). "CD5, CD10, and CD23 expression in Waldenstrom's macroglobulinemia". Clinical Lymphoma. 5 (4): 246–249. doi:10.3816/clm.2005.n.008. ISSN 1526-9655. PMID 15794857. Check date values in:
|date=(help) - ↑ Offit, K.; et al. (1992-11-15). "t(9;14)(p13;q32) denotes a subset of low-grade non-Hodgkin's lymphoma with plasmacytoid differentiation". Blood. 80 (10): 2594–2599. ISSN 0006-4971. PMID 1384792.
- ↑ Hirase, N.; et al. (2000-03). "Primary macroglobulinemia with t(11;18)(q21;q21)". Cancer Genetics and Cytogenetics. 117 (2): 113–117. doi:10.1016/s0165-4608(99)00156-9. ISSN 0165-4608. PMID 10704680. Check date values in:
|date=(help) - ↑ Chong, Y. Y.; et al. (1998-05). "A case of t(8;14) with total and partial trisomy 3 in Waldenstrom macroglobulinemia". Cancer Genetics and Cytogenetics. 103 (1): 65–67. doi:10.1016/s0165-4608(97)00346-4. ISSN 0165-4608. PMID 9595048. Check date values in:
|date=(help) - ↑ San Román, C.; et al. (1985-10). "Clonal abnormalities in patients with Waldenström's macroglobulinemia with special reference to a Burkitt-type t(8;14)". Cancer Genetics and Cytogenetics. 18 (2): 155–158. doi:10.1016/0165-4608(85)90065-2. ISSN 0165-4608. PMID 3931901. Check date values in:
|date=(help) - ↑ 25.0 25.1 25.2 25.3 25.4 Nguyen-Khac, Florence; et al. (2013-04). "Chromosomal aberrations and their prognostic value in a series of 174 untreated patients with Waldenström's macroglobulinemia". Haematologica. 98 (4): 649–654. doi:10.3324/haematol.2012.070458. ISSN 1592-8721. PMC 3659998. PMID 23065509. Check date values in:
|date=(help) - ↑ 26.0 26.1 Poulain, Stéphanie; et al. (2013-11). "Genome wide SNP array identified multiple mechanisms of genetic changes in Waldenstrom macroglobulinemia". American Journal of Hematology. 88 (11): 948–954. doi:10.1002/ajh.23545. ISSN 1096-8652. PMID 23861223. Check date values in:
|date=(help) - ↑ 27.0 27.1 27.2 Roccaro, Aldo M.; et al. (2009-04-30). "microRNA expression in the biology, prognosis, and therapy of Waldenström macroglobulinemia". Blood. 113 (18): 4391–4402. doi:10.1182/blood-2008-09-178228. ISSN 1528-0020. PMC 2943754. PMID 19074725.
- ↑ Sacco, Antonio; et al. (2016-06). "Epigenomics in Waldenstrom's macroglobulinaemia". Best Practice & Research. Clinical Haematology. 29 (2): 156–160. doi:10.1016/j.beha.2016.08.022. ISSN 1532-1924. PMID 27825461. Check date values in:
|date=(help) - ↑ 29.0 29.1 29.2 29.3 29.4 Treon, Steven P.; et al. (2020-04-10). "Genomic Landscape of Waldenström Macroglobulinemia and Its Impact on Treatment Strategies". Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 38 (11): 1198–1208. doi:10.1200/JCO.19.02314. ISSN 1527-7755. PMC 7351339 Check
|pmc=value (help). PMID 32083995 Check|pmid=value (help). - ↑ Hunter, Zachary R.; et al. (2016-08-11). "Transcriptome sequencing reveals a profile that corresponds to genomic variants in Waldenström macroglobulinemia". Blood. 128 (6): 827–838. doi:10.1182/blood-2016-03-708263. ISSN 1528-0020. PMC 4982454. PMID 27301862.
- ↑ Rodriguez, Sara; et al. (2022-01-21). "Preneoplastic somatic mutations including MYD88L265P in lymphoplasmacytic lymphoma". Science Advances. 8 (3): eabl4644. doi:10.1126/sciadv.abl4644. ISSN 2375-2548. PMC 8769557 Check
|pmc=value (help). PMID 35044826 Check|pmid=value (help). - ↑ 32.0 32.1 32.2 Sacco, Antonio; et al. (2017-05-23). "The importance of the genomic landscape in Waldenström's Macroglobulinemia for targeted therapeutical interventions". Oncotarget. 8 (21): 35435–35444. doi:10.18632/oncotarget.16130. ISSN 1949-2553. PMC 5471067. PMID 28423722.
- ↑ 33.0 33.1 Hunter, Zachary R.; et al. (2017-03-20). "Genomics, Signaling, and Treatment of Waldenström Macroglobulinemia". Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 35 (9): 994–1001. doi:10.1200/JCO.2016.71.0814. ISSN 1527-7755. PMID 28294689.
- ↑ 34.0 34.1 Hunter, Zachary R.; et al. (2018-11-13). "Insights into the genomic landscape of MYD88 wild-type Waldenström macroglobulinemia". Blood Advances. 2 (21): 2937–2946. doi:10.1182/bloodadvances.2018022962. ISSN 2473-9537. PMC 6234368. PMID 30401751.
- ↑ Poulain, Stéphanie; et al. (2013-05-30). "MYD88 L265P mutation in Waldenstrom macroglobulinemia". Blood. 121 (22): 4504–4511. doi:10.1182/blood-2012-06-436329. ISSN 1528-0020. PMID 23532735.
- ↑ Roccaro, Aldo M.; et al. (2014-06-26). "C1013G/CXCR4 acts as a driver mutation of tumor progression and modulator of drug resistance in lymphoplasmacytic lymphoma". Blood. 123 (26): 4120–4131. doi:10.1182/blood-2014-03-564583. ISSN 1528-0020. PMID 24711662.
- ↑ Kristinsson, Sigurdur Y.; et al. (2011-02). "What causes Waldenström's macroglobulinemia: genetic or immune-related factors, or a combination?". Clinical Lymphoma, Myeloma & Leukemia. 11 (1): 85–87. doi:10.3816/CLML.2011.n.015. ISSN 2152-2669. PMC 7020666 Check
|pmc=value (help). PMID 21454199. Check date values in:|date=(help) - ↑ Kristinsson, Sigurdur Y.; et al. (2008-10-15). "Risk of lymphoproliferative disorders among first-degree relatives of lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia patients: a population-based study in Sweden". Blood. 112 (8): 3052–3056. doi:10.1182/blood-2008-06-162768. ISSN 1528-0020. PMC 2569164. PMID 18703425.
- ↑ Ogmundsdóttir, H. M.; et al. (1999-08). "Enhanced B cell survival in familial macroglobulinaemia is associated with increased expression of Bcl-2". Clinical and Experimental Immunology. 117 (2): 252–260. doi:10.1046/j.1365-2249.1999.00971.x. ISSN 0009-9104. PMC 1905328. PMID 10444255. Check date values in:
|date=(help)
(use "Cite" icon at top of page)
Notes
*Primary authors will typically be those that initially create and complete the content of a page. If a subsequent user modifies the content and feels the effort put forth is of high enough significance to warrant listing in the authorship section, please contact the CCGA coordinators (contact information provided on the homepage). Additional global feedback or concerns are also welcome.