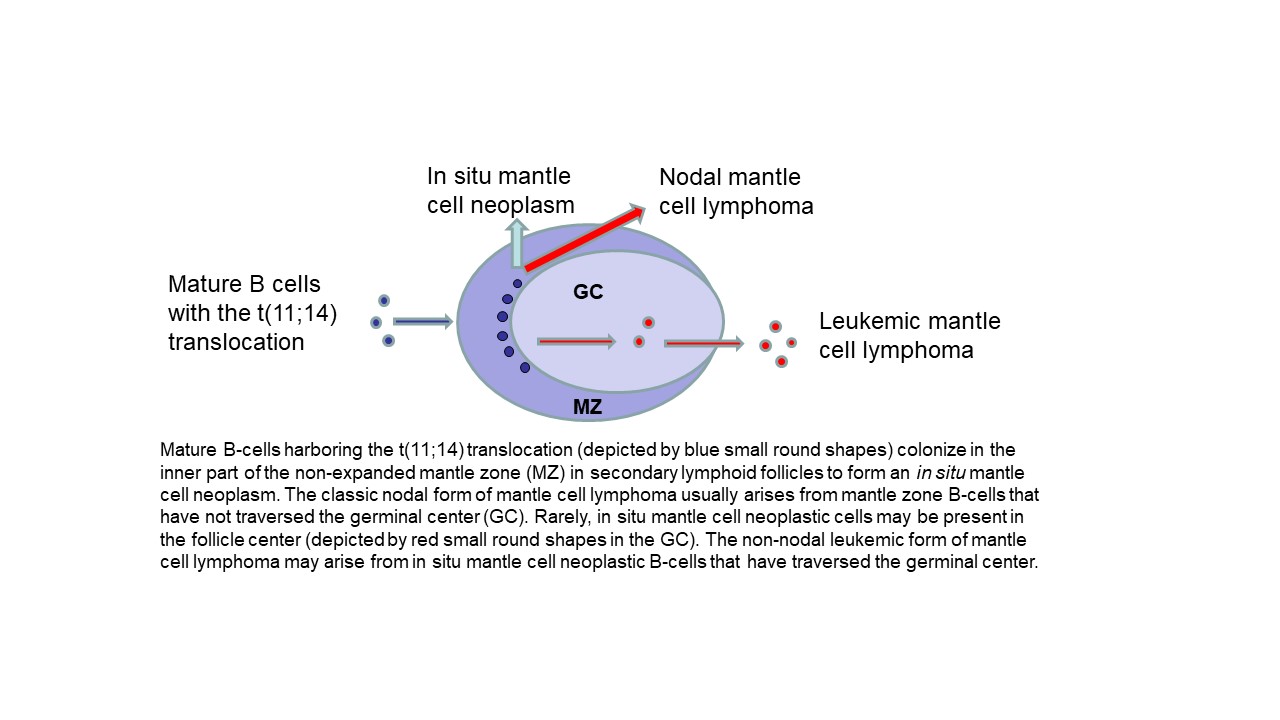
In situ mantle cell neoplasm
Haematolymphoid Tumours (5th ed.)
| This page is under construction |
editHAEM5 Conversion NotesThis page was converted to the new template on 2023-12-04. The original page can be found at HAEM4:In Situ Mantle Cell Neoplasia.
Primary Author(s)*
Rina Kansal, MD; Versiti Blood Center of Wisconsin
Cancer Category / Type
Mature B-cell neoplasm
Cancer Sub-Classification / Subtype
In situ mantle cell neoplasm[1]
Definition / Description of Disease
The Latin phrase “in situ” means “in the natural or original position or place”, as per the Merriam-Webster dictionary. In normal benign lymphoid tissues, the mantle zones of lymphoid follicles are formed by naïve mature B-cells after maturation from precursor B-cells in the bone marrow. In situ mantle cell neoplasm is a pre-malignant neoplasm composed of mantle cells “in their natural position” that harbor, in addition, the t(11;14) balanced translocation characteristic of mantle cell lymphoma with the overexpression of cyclin D1 protein.
An in situ mantle cell neoplasm may precede, co-exist with, or may occur after the development of an overt mantle cell lymphoma. This pre-malignant neoplasm is more often identified in patients newly diagnosed with an overt mantle cell lymphoma in whom a retrospective examination of a prior reactive-appearing lymph node or lymphoid tissue biopsy shows the presence of in situ mantle cell neoplasm. In situ mantle cell neoplasm is only rarely diagnosed in lymph nodes diagnosed as benign or reactive lymphoid hyperplasia after biopsy for enlargement or other symptomatic causes. The diagnostic criteria for in situ mantle cell neoplasm are currently based primarily on histopathologic examination (description in the morphologic features section). The pathologic diagnosis of in situ mantle cell neoplasm requires distinguishing from two major disease entities: (1) reactive lymphoid hyperplasia, and (2) mantle cell lymphoma with a mantle zone pattern. Of note, in situ mantle cell neoplasm may co-exist with any overt mature B-cell lymphoma, including as a component of a composite lymphoma.
Synonyms / Terminology
In situ mantle cell neoplasia, term used in the revised 4th edition World Health Organization classification[2] and in the International Consensus Classification of mature lymphoid neoplasms[3]; in situ mantle cell lymphoma (historical); mantle cell lymphoma in situ (historical); mantle cell lymphoma (MCL)-like B-cells of undetermined significance[4] (historical)
Epidemiology / Prevalence
The real prevalence of in situ mantle cell neoplasm is currently unknown. The published literature for in situ mantle cell neoplasm is limited primarily to case reports, including collectively studied cases,[5] and retrospective studies of cases of reactive lymphoid hyperplasia,[5][6] lymph nodes in specimens resected for cancer,[7] and lymph nodes and other organized lymphoid tissues resected for any non-hematologic cause prior to the diagnosis of overt mantle cell lymphoma[8].
| Study cohorts for detecting in situ mantle cell neoplasm | N patients studied | In situ mantle cell neoplasm, N cases detected in the study | ||
|---|---|---|---|---|
| Retrospective study of cyclin D1 immunohistochemical stains in 100 consecutive cases of lymphoid hyperplasia to detect in situ mantle cell neoplasm[5] | 100 | 0 (zero) by cyclin D1 stain[5] | ||
| 100 cases of reactive hyperplasia in lymph nodes studied by conventional cytogenetics[9] | 100 | 0 (zero) clonal chromosomal abnormalities by conventional cytogenetics[9] | ||
| Retrospective study of cyclin D1 immunohistochemical stains in reactive lymph nodes in surgical resection specimens of 131 consecutive patients with no history of lymphoma during a 3-month period[6] | 131 | 0 (zero) by cyclin D1 stain[6] | ||
| Retrospective study of lymph nodes (> 0.5 cm size) resected with cancer in 341 consecutive patients diagnosed with colorectal (n= 201) and breast carcinoma (n= 140) during 1998-2000[7] | 341 | 2 (0.58%) by cyclin D1 stain[7] | ||
| Retrospective study of previous resections of lymph nodes and organized lymphoid tissues due to non-hematologic indications for surgery in 126 patients with overt mantle cell lymphoma[8] | 126 | 2 (1.58%) by cyclin D1 stain[8] | ||
| Retrospective study of all morphologically reactive lymph nodes and benign-appearing extranodal lymphoid infiltrates predating lymphoma diagnosis in patients diagnosed with overt mantle cell lymphoma[6] | 37 | 0 (zero) cases reported with typical in situ mantle cell neoplasm by cyclin D1 stain;[6]
in 6 cases, minimal infiltration by cyclin D1 positive neoplastic mantle cells was identified at extranodal sites[6] |
Clinical Features
The clinical features at presentation or diagnosis may depend on the situations in which the in situ mantle cell neoplasm is diagnosed, including as follows:
- In a biopsy of an enlarged lymph node or extra-nodal lymphoid tissue: in biopsies performed with suspicion of a lymphoproliferative disease, in situ mantle cell neoplasm may co-exist with another mature B-cell lymphoma or with Castleman disease. Peripheral blood and/or bone marrow may be involved in addition to the histologic presence of in situ mantle cell neoplasm in lymphoid tissues.
- In previous biopsies retrospectively examined after the diagnosis of an overt mantle cell lymphoma.
- As an incidental finding in lymph nodes and lymphoid tissues examined for other causes, including lymph nodes in resected cancer specimens and lymphoid tissues in inflammatory conditions.
| Signs and Symptoms | In situ mantle cell neoplasm alone is asymptomatic and is therefore, found incidentally in lymph nodes and lymphoid tissues examined for other causes. However, since it may co-exist with an overt mantle cell lymphoma (nodal or leukemic), the signs and symptoms may vary according to the situation.
The presence of B-symptoms (weight loss, fever, night sweats), fatigue, or generalized lymphadenopathy should lead to the suspicion of an overt lymphoma. |
| Laboratory Findings | Similarly, there should be no cytopenias or lymphocytosis due to the presence of only an in situ mantle cell neoplasm.
Nevertheless, small or even minute populations of light chain restricted B-cells may be detected in peripheral blood by flow cytometric immunophenotyping in the absence of increased peripheral blood lymphocyte counts. In cases of composite lymphomas, flow cytometric immunophenotypic analysis of the lymph node or lymphoid tissues may show the presence of two neoplastic B-cell populations that may prompt further or retrospective histopathologic evaluation, including for cyclin D1 immunohistochemistry on tissue sections. |
The individual patient-level table below (with the preceding summary) shows the variability in clinical features and outcome among 31 previously reported patients diagnosed histologically with an in situ mantle cell neoplasm, along with the background in which in situ mantle cell neoplasia arose in these patients.
- Notably, 29% (9/31) of these in situ mantle cell neoplasm cases occurred in a background of a composite lymphoma comprised of another mature B-cell lymphoma, with follicular lymphoma (FL) being the most frequent, followed by chronic lymphocytic leukemia (CLL), and marginal zone lymphoma (nodal and extranodal types).
- Among the remaining 22 patients, 31.8% (7/22), 6 males and one female, developed an overt mantle cell lymphoma after 1-20 years. This development to an overt lymphoma occurred at 2y, 4y, 4y, 4y, 10y, and 20 y after a histologically diagnosable in situ mantle cell neoplasm for the initial overt mantle cell lymphoma and after 1 year for relapsed mantle cell lymphoma.
- An additional 22.7% (5/22) received chemotherapy or radiotherapy for lymphoma.
- An additional 9% (2/22) patients, both women, had leukemic involvement by mantle cell lymphoma and were alive with disease at long-term follow-up of 12 y and 19.5 y.
- One additional patient (1/22, 4.5%) died at 1.3 y.
- 27% (6/22) patients did not develop overt lymphoma at 0.08y, 0.66 y, 1 y, 3y, 5y, and 16 y follow-up.
- Follow-up not available for one (1/22) patient.
| Characteristics of reported patients and clinical situations for in situ mantle cell neoplasm diagnosis | Outcome after in situ mantle cell neoplasm diagnosis; follow-up time in years (y) | Composite lymphoma | Tissues with in situ mantle cell neoplasm | |
|---|---|---|---|---|
| 7 patients with in situ mantle cell neoplasm diagnosed with SOX11 positive neoplastic mantle cells[5] | ||||
| 43% (3 of 7) patients, all males, developed overt mantle cell lymphoma | ||||
| N=1 | Male aged 70 y with bilateral, small, palpable cervical lymph nodes, no ‘B’ symptoms or evidence of disease elsewhere; cervical lymph nodes with in situ mantle cell neoplasm[5][10] | Overt mantle cell lymphoma (MCL) developed at 4 y[5] | No | Cervical lymph nodes |
| N=1 | Male aged 65 y, in situ mantle cell neoplasm, retrospectively diagnosed in appendix excised for appendicitis[5][11] | Overt MCL developed at 4 y[5][11] | No | Appendix in appendicitis |
| N=1 | Male aged 66 y, previously diagnosed MCL treated with chemotherapy and obtained complete remission; three years later, underwent prostatectomy for prostate cancer and the pelvic lymph nodes with that surgery were retrospectively examined later to reveal in situ mantle cell neoplasm[5] | Overt relapsed MCL in inguinal lymph nodes at 1 y[5] | No | Pelvic lymph nodes with prostatectomy for cancer |
| 28.5% (2 of 7) patients, both females, received chemotherapy | ||||
| N=1 | Female aged 42 y, with multiple lymph node sites (axillary, cervical, inguinal, iliac) and gastrointestinal tract involved; in situ mantle cell neoplasm in enlarged lymph nodes; BM and PB not involved[11] | Received chemotherapy; alive with no disease at 6 y[11] | No | Axillary and inguinal lymph nodes and gastrointestinal tract biopsied |
| N=1 | Female aged 65 y, with in situ mantle cell neoplasm in enlarged lymph nodes, BM involved[5] | Received chemotherapy; alive with no disease at 0.5 y[5] | No | Lymph nodes, site not specified, with non-specific granulomas |
| 28.5% (2 of 7) patients, both males, given active surveillance until follow-up time | ||||
| N=1 | Male aged 68 y, with in situ mantle cell neoplasm in enlarged mediastinal lymph nodes | Not treated
Alive with no lymphoma at 1 y |
No | Mediastinal lymph node |
| N=1 | Male aged 82 y, with previous chronic lymphocytic leukemia (CLL); in situ mantle cell neoplasm in enlarged oropharyngeal lymphoid tissue and peripheral blood involvement by MCL and CLL | Not treated
Alive with disease at 3 y |
Yes, with CLL | Oropharyngeal tissue |
| 1 patient with in situ mantle cell neoplasm, SOX11 not performed[5] | ||||
| N=1 | Male aged 82 y, with CLL and in situ mantle cell neoplasm in an enlarged lymph node | Received chemotherapy; alive at 1.5 y | Yes, with CLL | Lymph node |
| 9 patients with in situ mantle cell neoplasm diagnosed with SOX11 negative neoplastic mantle cells[5] | ||||
| 22% (2 of 9) patients, both females, with leukemic involvement of peripheral blood (PB) and bone marrow (BM) by MCL[5] | ||||
| N=1 | Female aged 29 y, with in situ mantle cell neoplasm in enlarged lymph nodes and leukemic involvement of peripheral blood and bone marrow[5] | Active surveillance
Leukemic involvement by MCL; alive with disease at 19.5 y[5] |
No | Axillary lymph node |
| N=1 | Female aged 70 y, presented in December 1999 with a slowly growing submandibular lymph node over 18 months; biopsy showed non-necrotizing granulomatous lymphadenitis with in situ mantle cell neoplasm; cytogenetics on lymph node tissue showed t(11;14)(q13;q32); PB and BM involved by leukemic mantle cells[5][9] | Active surveillance
Leukemic involvement by MCL; alive with disease at 12 y[5] |
No | Submandibular lymph node with non-necrotizing granulomata |
| 33% patients (3 of 9), 2 males and 1 female, treated with chemotherapy or radiotherapy[5] | ||||
| N=1 | Male aged 42 y, with Castleman disease, hyaline vascular type[5] | Treated with radiotherapy, alive at 1.7 y | No | Supraclavicular lymph node with Castleman disease, hyaline vascular type |
| N=1 | Male aged 58 y, clinical situation not known except for intestinal biopsy and bone marrow involved by neoplastic mantle cells[5] | Treated with chemotherapy, alive at 1.4 y | No | Intestine |
| N=1 | Female aged 78 y with breast cancer treated by mastectomy 37 y prior to presentation with an enlarged posterior neck lymph node excised to show a composite lymphoma with in situ mantle cell neoplasm[5][12] | Treated with radiotherapy, alive at 2 y | Yes, with nodal marginal zone lymphoma (MZL) | Posterior cervical lymph node |
| Remaining 4 patients with SOX11 negative in situ mantle cell neoplasm[5] | ||||
| N=1 | Female aged 84 y with history of rectal carcinoma treated 5 y prior to splenectomy, which was performed due to multiple masses detected radiologically in the spleen; a composite lymphoma in the spleen with follicular lymphoma (FL), in situ follicular B-cell neoplasm and in situ mantle cell neoplasm [13] | Refused treatment; intra-abdominal lymph nodes enlarged 9 months after splenectomy
Dead at 13 months (1.1 y) of unknown cause[13] |
Yes, with FL and in situ follicular B-cell neoplasm[13][1] | Spleen |
| N=1 | Female aged 78 y, diagnosed with in situ mantle cell neoplasm in a composite lymphoma in an enlarged lacrimal gland with extranodal MZL and in situ mantle cell neoplasm[5] | No follow-up available | Yes, with extranodal MZL | Lacrimal gland |
| N=1 | Male aged 59 y, with papillary thyroid cancer and in situ mantle cell neoplasm in cervical lymph nodes[5] | Alive with no lymphoma at 5 y | No | Cervical lymph nodes with papillary thyroid carcinoma |
| N=1 | Female aged 42 y, with breast cancer and nodal involvement by in situ mantle cell neoplasm[5] | Alive with no lymphoma at 1 y | No | |
| Other reported patients with in situ mantle cell neoplasm | ||||
| N=1 | Male aged 45 y, rectal biopsies due to bleeding per rectum diagnosed as benign colonic mucosa; Retrospective diagnosis of in situ mantle cell neoplasm made in the original rectal biopsy after overt MCL diagnosed 2 y later; after chemotherapy, residual lymphoid aggregates in colonic biopsies showed features similar to the original in situ mantle cell neoplasm with cyclin D1 positive and neoplastic cells positive for t(11;14) by FISH[14] | Overt, disseminated MCL at 2 y; at that time, patient presented with ileocecal intussusception due to enlarged lymph nodes | No | Rectal biopsy, original, and colonic biopsies after chemotherapy |
| N=1 | Female aged 71 y with overt MCL, clinical stage 4A, in 2013; retrospective review of previous lymph nodes excised with invasive ductal breast carcinoma in 2003 (ten y prior to the overt MCL) showed in situ mantle cell neoplasm[8] | Overt MCL 10 y after in situ neoplasm; Dead 1 year after the diagnosis of overt MCL (or 11 y after diagnosis of in situ mantle cell neoplasm) | No | Lymph nodes excised with invasive ductal breast carcinoma; retrospective diagnosis |
| N=1 | Male aged 71 y, diagnosed with colonic adenocarcinoma with metastatic carcinoma in resected lymph nodes and retrospectively diagnosed in situ mantle cell neoplasm in a pericolonic lymph node 4 years later[15] | Overt MCL at 4 y diagnosed by biopsy of enlarged preauricular lymph node/parotid mass and BM; received chemotherapy; relapsed MCL 2 y post-chemotherapy | No | Pericolonic lymph node with metastatic colon adenocarcinoma |
| N=1 | Male aged 68 y, retrospective diagnosis of in situ mantle cell neoplasm in mesenteric lymph nodes resected with colorectal carcinoma; case identified in a systematic study as an incidental finding[7] | Dead at 1.33 y (16 months), no lymphoma | No | Mesenteric lymph nodes with colorectal cancer;
retrospective diagnosis |
| N=1 | Female aged 42 y, inguinal mass, imaging showed enlarged cervical, axillary, iliac, and inguinal lymph nodes, clinically staged as 4A; biopsy of inguinal and axillary lymph nodes showed a similar pattern in both lymph nodes of marked follicular hyperplasia and in situ mantle cell neoplasm[11] | Chemotherapy given; further follow-up not available | No | Inguinal and axillary lymph nodes with lymphoid hyperplasia |
| N=1 | Female aged 46 y with breast cancer, retrospective diagnosis of in situ mantle cell neoplasm in axillary lymph nodes; case identified in a systematic study as an incidental finding[7] | Alive at 16 y (192 months), no lymphoma | No | Axillary lymph nodes in breast cancer; retrospective diagnosis |
| N=1 | Male aged 79 y, overt MCL with clinical stage 1A in 2008, retrospective review of lymph nodes excised with malignant melanoma in 1988 (20 y prior to overt MCL) showed in situ mantle cell neoplasm[8] | Overt MCL 20 y after in situ mantle cell neoplasm; Alive with lymphoma at the time of publication in 2015 (about 7 y from overt MCL) | No | Lymph nodes excised with malignant melanoma, retrospective |
| N=1 | Male with age in 40’s, with stage 4B follicular lymphoma (FL) diagnosed and treated 6 y before presentation with generalized lymphadenopathy at relapse; cervical lymph node at relapse showed a composite lymphoma composed of FL and in situ mantle cell neoplasm[10] | No follow-up available | Yes, with FL | Cervical lymph node |
| N=1 | Female aged 76 y, clinical suspicion of lymphoma, previous history of right cheek squamous cell carcinoma; right cervical lymph node showed follicular lymphoma and in situ mantle cell neoplasm[7] | Alive at 0.75 y (9 months), in remission | Yes, with FL | Right cervical lymph node with previous history of right cheek carcinoma |
| N=1 | Male aged 56 y, generalized lymphadenopathy, inguinal lymph node showed composite lymphoma (FL and in situ mantle cell lymphoma); cyclin D1 positive neoplastic mantle cells;
Flow cytometric analysis of lymph node tissue showed the neoplastic mantle cells to express bright surface kappa light chain immunoglobulin while the FL cells expressed less bright kappa light chain immunoglobulin[16] |
Received chemotherapy, in complete remission and alive at 27 months (2.25 y)[17] | Yes, with FL | Inguinal lymph node biopsy |
| N=1 | Male aged 76 y, recent diagnosis of prostate carcinoma, with generalized lymphadenopathy, including in the axilla and mediastinum; axillary lymph node biopsy showed composite lymphoma with FL and in situ mantle cell neoplasm; BM and gastric biopsy involved by FL[18] | Not available | Yes, with FL | Axillary lymph node in a patient with recent diagnosis of prostate carcinoma |
| N=1 | Female aged 70 y, nasopharyngeal biopsy with lymphoid hyperplasia and situ mantle cell neoplasm; biopsy repeated at 3 y showed similar in situ mantle cell neoplasm[19] | Alive with no progression to overt lymphoma at 3 y from first biopsy | No | Nasopharyngeal biopsy with lymphoid hyperplasia |
| N=1 | Female aged 31 y, cervical lymphadenopathy with Castleman disease, hyaline vascular type, with in situ mantle cell neoplasm[20] | Alive with no lymphoma at 0.66 y (8 months) | No | Cervical lymph node with Castleman disease, hyaline vascular type |
| N=1 | Male aged 73 y, lymphadenopathy with longstanding 20 y history of psoriasis; axillary lymph node showed dermatopathic lymphadenitis in conjunction with human herpes simplex virus 8 (HHV8) positive multicentric Castleman disease of mixed type and in situ mantle cell neoplasm[21] | Treated with Rituximab followed by antibiotics for Staphylococcal infection at the time of publication in 2021 | No | Axillary lymph node with dermatopathic lymphadenitis and HHV8 positive multicentric Castleman disease |
Sites of Involvement
Nodal and extranodal: virtually any lymphoid tissue sites in the body may be involved.
Bone marrow (BM) and peripheral blood (PB) may be involved by leukemic mantle cell lymphoma in patients with a histologic diagnosis of in situ mantle cell neoplasm in a lymph node or lymphoid tissue. In those cases, BM or PB involvement by neoplastic mantle cell lymphoma cells would be considered leukemic involvement (and not “in situ mantle cell neoplasm” in PB or BM).
Morphologic Features
In situ mantle cell neoplasm is characterized by the presence of cyclin D1 positive, SOX11 positive or negative, CD5 positive or negative, CD20 positive neoplastic B-cells in unexpanded mantle zones surrounding follicle centers in lymph nodes or extranodal lymphoid tissues. Rarely, the neoplastic mantle cells may also be present in the follicle center and identified only by immunohistochemical stains. The nodal architecture is preserved and is typically reactive-appearing except for the presence of the neoplastic in situ mantle cells that are identified by immunohistochemical staining for cyclin D1. The diagnosis can be difficult or may not even be possible to render solely by hematoxylin and eosin (H&E) stain morphology.
In situ mantle cell neoplasm must be differentiated from overt mantle cell lymphoma with a mantle zone pattern. In contrast with an in situ mantle cell neoplasm, overt mantle cell lymphoma may show any of the following features: greater follicle density than in a reactive lymph node, focal obliteration of nodal architecture, focally fused mantle zones, interfollicular neoplastic mantle cell nodules, and expanded mantle zones with monotonous or densely packed neoplastic mantle cells with slight to moderately irregular nuclear contours, slightly more open nuclear chromatin, inconspicuous nucleoli and scant cytoplasm.
Notably, in mantle cell lymphoma with a mantle zone pattern, the lymph node architecture may also be preserved, as reported.[22] In those two cases, a high index of suspicion due to monotonous mantle cells with slight nuclear irregularity and the clinical history of lymphadenopathy at other sites led to a cyclin D1 stain and the accurate diagnosis of an overt mantle cell lymphoma.[22]
Immunophenotype
Immunohistochemistry for cyclin D1 is required for the diagnosis of in situ mantle cell neoplasm in virtually all cases. The neoplastic cells are CD20 positive B-cells that co-express cyclin D1.
Theoretically, cyclin D1 negative mantle cell lymphoma may also have an in situ neoplastic component but that has not yet been reported.
| Finding | Marker |
|---|---|
| Neoplastic cells Positive (universal) | CD20, cyclin D1 |
| Neoplastic cells Positive (subset of cases) | CD5, SOX11 |
| Neoplastic cells Negative (universal) | CD3 |
| Neoplastic cells Negative (subset of cases) | CD5, SOX11 |
Chromosomal Rearrangements (Gene Fusions)
The balanced translocation t(11;14)(q13;q32) is characteristic of mantle cell lymphoma and was identified in all cases of histologically identified in situ mantle cell neoplasm (whenever it was examined for in addition to the overexpression of cyclin D1 protein).
| Chromosomal Rearrangement | Genes in Fusion (5’ or 3’ Segments) | Pathogenic Derivative | Prevalence | Diagnostic Significance (Yes, No or Unknown) | Prognostic Significance (Yes, No or Unknown) | Therapeutic Significance (Yes, No or Unknown) | Notes |
|---|---|---|---|---|---|---|---|
| t(11;14)(q13;q32) | CCND1::IGH | Yes | Not yet known, if any, prognostic significance of t(11;14) in in situ mantle cell neoplasm;
a subset of mantle cell lymphoma with the t(11;14) may have good prognosis[23] |
Not yet known, if any | The t(11;14) is characteristic of in situ mantle cell neoplasm and overt mantle cell lymphoma in the appropriate morphology and clinical context. However, this translocation may also be present in other lymphoid neoplasms and has also been identified in healthy individuals at a lower incidence than the t(14;18) translocation in healthy individuals.[24][25][26] |
Individual Region Genomic Gain / Loss / LOH
These have not been studied specifically for in situ mantle cell neoplasm.
| Chr # | Gain / Loss / Amp / LOH | Minimal Region Genomic Coordinates [Genome Build] | Minimal Region Cytoband | Diagnostic Significance (Yes, No or Unknown) | Prognostic Significance (Yes, No or Unknown) | Therapeutic Significance (Yes, No or Unknown) | Notes |
|---|---|---|---|---|---|---|---|
| EXAMPLE
7 |
EXAMPLE Loss | EXAMPLE
chr7:1- 159,335,973 [hg38] |
EXAMPLE
chr7 |
Yes | Yes | No | EXAMPLE
Presence of monosomy 7 (or 7q deletion) is sufficient for a diagnosis of AML with MDS-related changes when there is ≥20% blasts and no prior therapy (add reference). |
| EXAMPLE
8 |
EXAMPLE Gain | EXAMPLE
chr8:1-145,138,636 [hg38] |
EXAMPLE
chr8 |
No | No | No | EXAMPLE
Common recurrent secondary finding for t(8;21) (add reference). |
Characteristic Chromosomal Patterns
Put your text here (EXAMPLE PATTERNS: hyperdiploid; gain of odd number chromosomes including typically chromosome 1, 3, 5, 7, 11, and 17; co-deletion of 1p and 19q; complex karyotypes without characteristic genetic findings; chromothripsis)
| Chromosomal Pattern | Diagnostic Significance (Yes, No or Unknown) | Prognostic Significance (Yes, No or Unknown) | Therapeutic Significance (Yes, No or Unknown) | Notes |
|---|---|---|---|---|
| EXAMPLE
Co-deletion of 1p and 18q |
Yes | No | No | EXAMPLE:
See chromosomal rearrangements table as this pattern is due to an unbalanced derivative translocation associated with oligodendroglioma (add reference). |
Gene Mutations (SNV / INDEL)
There are numerous publications for gene mutations in overt mantle cell lymphoma. A recent systematic meta-analysis of 32 studies published during 2006 to 2019, excluding review articles, detailed the findings for gene mutations in mantle cell lymphoma by analyzing 2127 individual overt mantle cell lymphoma patients and 2173 samples that were included in the analyzed studies.[27] These studies included the nodal and the leukemic forms of overt mantle cell lymphoma. As per this meta-analysis, in overt mantle cell lymphoma tumor or bone marrow samples at diagnosis or baseline, the most frequent genetic abnormalities occurred in the ATM (43.5%), TP53 (26.8%), CDKN2A (23.9%), CCND1 (20.2%), NSD2 (15.0%), KMT2A (8.9%), S1PR1 (8.6%), and CARD11 (8.5%) genes.[27] Aberrations in IGH (38.4%) and MYC (20.8%) were detected primarily through cytogenetic methods, also in those same tumor specimens.[27]
However, there have not yet been any studies that have examined in situ mantle cell neoplasm (without an overt lymphoma) for gene mutations.
| Gene; Genetic Alteration | Presumed Mechanism (Tumor Suppressor Gene [TSG] / Oncogene / Other) | Prevalence (COSMIC / TCGA / Other) | Concomitant Mutations | Mutually Exclusive Mutations | Diagnostic Significance (Yes, No or Unknown) | Prognostic Significance (Yes, No or Unknown) | Therapeutic Significance (Yes, No or Unknown) | Notes |
|---|---|---|---|---|---|---|---|---|
| EXAMPLE: TP53; Variable LOF mutations
EXAMPLE: EGFR; Exon 20 mutations EXAMPLE: BRAF; Activating mutations |
EXAMPLE: TSG | EXAMPLE: 20% (COSMIC)
EXAMPLE: 30% (add Reference) |
EXAMPLE: IDH1 R123H | EXAMPLE: EGFR amplification | EXAMPLE: Excludes hairy cell leukemia (HCL) (add reference).
|
Note: A more extensive list of mutations can be found in cBioportal (https://www.cbioportal.org/), COSMIC (https://cancer.sanger.ac.uk/cosmic), ICGC (https://dcc.icgc.org/) and/or other databases. When applicable, gene-specific pages within the CCGA site directly link to pertinent external content.
Epigenomic Alterations
Epigenomic alterations have been studied in overt mantle cell lymphoma.[28] In situ mantle cell neoplasia has not yet been studied.
Genes and Main Pathways Involved
In those individuals without an overt lymphoma, in situ mantle cell neoplasm is often considered to be, or might be similar to being a tissue counterpart of rare circulating t(11:14) positive lymphocytes in peripheral blood. While overt mantle cell lymphoma involves dysregulated cell cycle and DNA damage response pathways, the steps of the development of an overt mantle cell lymphoma from an in situ mantle cell neoplasm are not yet understood.
| Gene; Genetic Alteration | Pathway | Pathophysiologic Outcome |
|---|---|---|
| EXAMPLE: BRAF and MAP2K1; Activating mutations | EXAMPLE: MAPK signaling | EXAMPLE: Increased cell growth and proliferation |
| EXAMPLE: CDKN2A; Inactivating mutations | EXAMPLE: Cell cycle regulation | EXAMPLE: Unregulated cell division |
| EXAMPLE: KMT2C and ARID1A; Inactivating mutations | EXAMPLE: Histone modification, chromatin remodeling | EXAMPLE: Abnormal gene expression program |
Genetic Diagnostic Testing Methods
Fluorescence in situ hybridization (FISH) for the t(11;14) translocation is the most commonly used method, in conjunction with immunohistochemistry for the overexpression of cyclin D1 in the neoplastic mantle cells. Both FISH and immunohistochemistry may be performed on paraffin-embedded tissue sections to allow identification of the abnormalities within specific cells (neoplastic) in the histologic sections.
Conventional cytogenetics performed on involved lymphoid tissues is also used by some laboratories.[9]
Familial Forms
Overt mantle cell lymphoma has been reported to occur in families with other family members having lymphoproliferative disorders, including chronic lymphocytic leukemia. These reports are limited to small studies and case reports of overt mantle cell lymphoma, with yet unexplained or rarely explained genetic basis for the familial occurrence of overt mantle cell lymphoma in these families. Conversely, rare cases of overt mantle cell lymphoma with germline mutations have been reported but the clinical and family history are usually not available in these reported cases wherein tumor specimens were examined, with or without germline specimen analysis.
Nevertheless, an in situ mantle cell neoplasm has not been reported to occur in a familial manner or in any of the reported overt mantle cell lymphoma cases with an identifed germline alteration.
The incidence and prevalence of familial overt mantle cell lymphoma are currently unknown. Also to note is that earlier instances of familial chronic lymphoproliferative disorders that included chronic lymphocytic leukemia might have included cases of mantle cell lymphoma. Specifically, the analysis for familial risk among 153,115 Swedish patients with hematologic malignancies included 18,521 patients with chronic lymphocytic leukemia (CLL), including 8,043 (43.4%; 8043/18521) CLL patients diagnosed during the same time period when mantle cell lymphoma was not yet diagnosed.[29]
In one Spanish study of 85 patients with overt mantle cell lymphoma, 2 (2.4%) patients with overt mantle cell lymphoma were identified that had first degree relatives with another lymphoproliferative disorder.[30] A third overt mantle cell lymphoma patient was reported in the same publication with familial lymphoproliferative neoplasms.[30] One of those 3 patients was diagnosed as mantle cell lymphoma after re-review of previous pathology, with a previous diagnosis of chronic lymphocytic leukemia.[30]
In another study of 109 patients with hematologic malignancies, one case of overt mantle cell lymphoma among 7 examined cases of non-Hodgkin lymphomas was identified to harbor a 15 bp deletion in the CHEK2 gene both in the overt tumor sample and in the germline.[31] Similarly, a heterozygous germline abnormality in the ATM gene was found in 1 of 4 normal tissue samples associated with 4 overt MCL tumor specimens in one study;[32] in a recent whole genome sequencing study of overt mantle cell lymphoma tumor and normal specimens, germline mutations in ATM and CHEK2 genes were reported in 7 and 2 cases, respectively.[28] A personal history of cancer other than mantle cell lymphoma or a family history of cancer was unavailable for these cases.[28][31][32]
Notably, the germline basis of familial mantle cell lymphoma was recently described in one Chinese patient with maternally inherited Lynch syndrome (with co-segregation of a MLH1 variant), paternal history of a diffuse large B-cell lymphoma in the father, and follicular lymphoma in one sibling.[33] The index patient with overt mantle cell lymphoma also developed a colonic adenocarcinoma due to mismatch repair defects.[33]
Additional Information
Put your text here
Links
HAEM5:Leukaemic non-nodal mantle cell lymphoma
HAEM5:Chronic lymphocytic leukaemia/small lymphocytic lymphoma
National Comprehensive Cancer Network (NCCN) Guidelines. B-cell Lymphomas. Version 5.2022 — July 12, 2022. Accessed July 25, 2022[1]
References
- ↑ 1.0 1.1 Alaggio, Rita; et al. (2022-07). "The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms". Leukemia. 36 (7): 1720–1748. doi:10.1038/s41375-022-01620-2. ISSN 1476-5551. PMC PMC9214472 Check
|pmc=value (help). PMID 35732829 Check|pmid=value (help). Check date values in:|date=(help)CS1 maint: PMC format (link) - ↑ Swerdlow SH, et al., (2017). Mantle cell lymphoma, in World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues, Revised 4th edition. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Arber DA, Hasserjian RP, Le Beau MM, Orazi A, and Siebert R, Editors. IARC Press: Lyon, France, p290.
- ↑ Campo, Elias; et al. (2022-06-02). "The International Consensus Classification of Mature Lymphoid Neoplasms: A Report from the Clinical Advisory Committee". Blood: blood.2022015851. doi:10.1182/blood.2022015851. ISSN 1528-0020. PMID 35653592 Check
|pmid=value (help). - ↑ Fend, Falko; et al. (2012-09). "Early lesions in lymphoid neoplasia: Conclusions based on the Workshop of the XV. Meeting of the European Association of Hematopathology and the Society of Hematopathology, in Uppsala, Sweden". Journal of Hematopathology. 5 (3). doi:10.1007/s12308-012-0148-6. ISSN 1868-9256. PMC 3845020. PMID 24307917. Check date values in:
|date=(help) - ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 5.17 5.18 5.19 5.20 5.21 5.22 5.23 5.24 5.25 5.26 5.27 Carvajal-Cuenca, Alejandra; et al. (2012-02). "In situ mantle cell lymphoma: clinical implications of an incidental finding with indolent clinical behavior". Haematologica. 97 (2): 270–278. doi:10.3324/haematol.2011.052621. ISSN 1592-8721. PMC 3269489. PMID 22058203. Check date values in:
|date=(help) - ↑ 6.0 6.1 6.2 6.3 6.4 6.5 Adam, Patrick; et al. (2012-12). "Incidence of preclinical manifestations of mantle cell lymphoma and mantle cell lymphoma in situ in reactive lymphoid tissues". Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc. 25 (12): 1629–1636. doi:10.1038/modpathol.2012.117. ISSN 1530-0285. PMID 22790016. Check date values in:
|date=(help) - ↑ 7.0 7.1 7.2 7.3 7.4 7.5 Bermudez, Glenda; et al. (2016-07). "Incidental and Isolated Follicular Lymphoma In Situ and Mantle Cell Lymphoma In Situ Lack Clinical Significance". The American Journal of Surgical Pathology. 40 (7): 943–949. doi:10.1097/PAS.0000000000000628. ISSN 1532-0979. PMID 26945339. Check date values in:
|date=(help) - ↑ 8.0 8.1 8.2 8.3 8.4 Teixeira Mendes, Larissa Sena; et al. (2016-02). "The relationship between overt and in-situ lymphoma: a retrospective study of follicular and mantle cell lymphoma cases". Histopathology. 68 (3): 461–463. doi:10.1111/his.12753. ISSN 1365-2559. PMID 26052648. Check date values in:
|date=(help) - ↑ 9.0 9.1 9.2 9.3 Espinet, Blanca; et al. (2005-11). "Clonal proliferation of cyclin D1-positive mantle lymphocytes in an asymptomatic patient: an early-stage event in the development or an indolent form of a mantle cell lymphoma?". Human Pathology. 36 (11): 1232–1237. doi:10.1016/j.humpath.2005.08.021. ISSN 0046-8177. PMID 16260278. Check date values in:
|date=(help) - ↑ 10.0 10.1 Aqel, N.; et al. (2008-01). "In-situ mantle cell lymphoma--a report of two cases". Histopathology. 52 (2): 256–260. doi:10.1111/j.1365-2559.2007.02906.x. ISSN 1365-2559. PMID 18184277. Check date values in:
|date=(help) - ↑ 11.0 11.1 11.2 11.3 11.4 Bassarova, Assia; et al. (2008-10). "Mantle cell lymphoma with partial involvement of the mantle zone: an early infiltration pattern of mantle cell lymphoma?". Virchows Archiv: An International Journal of Pathology. 453 (4): 407–411. doi:10.1007/s00428-008-0621-x. ISSN 0945-6317. PMID 18696109. Check date values in:
|date=(help) - ↑ Rodig, Scott J.; et al. (2006-11). "Mantle cell lymphoma arising within primary nodal marginal zone lymphoma: a unique presentation of two uncommon B-cell lymphoproliferative disorders". Cancer Genetics and Cytogenetics. 171 (1): 44–51. doi:10.1016/j.cancergencyto.2006.06.018. ISSN 0165-4608. PMID 17074590. Check date values in:
|date=(help) - ↑ 13.0 13.1 13.2 Roullet, Michele R.; et al. (2010-04). "Coexisting follicular and mantle cell lymphoma with each having an in situ component: A novel, curious, and complex consultation case of coincidental, composite, colonizing lymphoma". American Journal of Clinical Pathology. 133 (4): 584–591. doi:10.1309/AJCP5RT4MRSDGKSX. ISSN 1943-7722. PMID 20231612. Check date values in:
|date=(help) - ↑ Neto, Antonio G.; et al. (2012-12). "Colonic in situ mantle cell lymphoma". Annals of Diagnostic Pathology. 16 (6): 508–514. doi:10.1016/j.anndiagpath.2011.05.001. ISSN 1532-8198. PMID 21840231. Check date values in:
|date=(help) - ↑ Edlefsen, Kerstin L.; et al. (2011-08). "Early lymph node involvement by mantle cell lymphoma limited to the germinal center: report of a case with a novel "follicular in situ" growth pattern". American Journal of Clinical Pathology. 136 (2): 276–281. doi:10.1309/AJCP6KFFGTC8PLVR. ISSN 1943-7722. PMID 21757601. Check date values in:
|date=(help) - ↑ Demurtas, Anna; et al. (2011-04). "Usefulness of multiparametric flow cytometry in detecting composite lymphoma: study of 17 cases in a 12-year period". American Journal of Clinical Pathology. 135 (4): 541–555. doi:10.1309/AJCPQKE25ADCFZWN. ISSN 1943-7722. PMID 21411776. Check date values in:
|date=(help) - ↑ Papathomas, Thomas G.; et al. (2012-04). "Mantle cell lymphoma as a component of composite lymphoma: clinicopathologic parameters and biologic implications". Human Pathology. 43 (4): 467–480. doi:10.1016/j.humpath.2011.08.024. ISSN 1532-8392. PMID 22221705. Check date values in:
|date=(help) - ↑ Subtil, Antonio; et al. (2019-05-30). "Follicular lymphoma with composite in situ mantle cell neoplasia". Blood. 133 (22): 2460. doi:10.1182/blood.2019000012. ISSN 1528-0020. PMID 31147376.
- ↑ Koletsa, Triantafyllia; et al. (2013-11). "In situ mantle cell lymphoma in the nasopharynx". Head & Neck. 35 (11): E333–337. doi:10.1002/hed.23206. ISSN 1097-0347. PMID 23280758. Check date values in:
|date=(help) - ↑ Dobrea, Camelia; et al. (2011). ""In situ" mantle cell lymphoma associated with hyaline-vascular Castleman disease". Romanian Journal of Morphology and Embryology = Revue Roumaine De Morphologie Et Embryologie. 52 (3 Suppl): 1147–1151. ISSN 2066-8279. PMID 22119840.
- ↑ Zanelli, Magda; et al. (2021-06-24). "HHV8-Positive Castleman Disease and In Situ Mantle Cell Neoplasia within Dermatopathic Lymphadenitis, in Longstanding Psoriasis". Diagnostics (Basel, Switzerland). 11 (7): 1150. doi:10.3390/diagnostics11071150. ISSN 2075-4418. PMC 8305231 Check
|pmc=value (help). PMID 34202434 Check|pmid=value (help). - ↑ 22.0 22.1 Richard, P.; et al. (2006-09). ""In situ-like" mantle cell lymphoma: a report of two cases". Journal of Clinical Pathology. 59 (9): 995–996. doi:10.1136/jcp.2005.030783. ISSN 0021-9746. PMC 1860464. PMID 16935977. Check date values in:
|date=(help) - ↑ Orchard, Jenny; et al. (2003-06-15). "A subset of t(11;14) lymphoma with mantle cell features displays mutated IgVH genes and includes patients with good prognosis, nonnodal disease". Blood. 101 (12): 4975–4981. doi:10.1182/blood-2002-06-1864. ISSN 0006-4971. PMID 12609845.
- ↑ Hirt, Carsten; et al. (2004-08-01). "Low prevalence of circulating t(11;14)(q13;q32)-positive cells in the peripheral blood of healthy individuals as detected by real-time quantitative PCR". Blood. 104 (3): 904–905. doi:10.1182/blood-2004-02-0738. ISSN 0006-4971. PMID 15265798.
- ↑ Nambiar, Mridula; et al. (2010-01). "Prevalence and analysis of t(14;18) and t(11;14) chromosomal translocations in healthy Indian population". Annals of Hematology. 89 (1): 35–43. doi:10.1007/s00277-009-0755-1. ISSN 1432-0584. PMID 19488754. Check date values in:
|date=(help) - ↑ Lecluse, Y.; et al. (2009-06). "t(11;14)-positive clones can persist over a long period of time in the peripheral blood of healthy individuals". Leukemia. 23 (6): 1190–1193. doi:10.1038/leu.2009.31. ISSN 1476-5551. PMID 19242498. Check date values in:
|date=(help) - ↑ 27.0 27.1 27.2 Hill, Holly A.; et al. (2020-07-14). "Genetic mutations and features of mantle cell lymphoma: a systematic review and meta-analysis". Blood Advances. 4 (13): 2927–2938. doi:10.1182/bloodadvances.2019001350. ISSN 2473-9537. PMC 7362354 Check
|pmc=value (help). PMID 32598477 Check|pmid=value (help). - ↑ 28.0 28.1 28.2 Nadeu, Ferran; et al. (2020-09-17). "Genomic and epigenomic insights into the origin, pathogenesis, and clinical behavior of mantle cell lymphoma subtypes". Blood. 136 (12): 1419–1432. doi:10.1182/blood.2020005289. ISSN 1528-0020. PMC 7498364 Check
|pmc=value (help). PMID 32584970 Check|pmid=value (help). - ↑ Sud, Amit; et al. (2019-09-19). "Analysis of 153 115 patients with hematological malignancies refines the spectrum of familial risk". Blood. 134 (12): 960–969. doi:10.1182/blood.2019001362. ISSN 1528-0020. PMC 6789511. PMID 31395603.
- ↑ 30.0 30.1 30.2 Tort, Frederic; et al. (2004-03). "Familial lymphoid neoplasms in patients with mantle cell lymphoma". Haematologica. 89 (3): 314–319. ISSN 1592-8721. PMID 15020270. Check date values in:
|date=(help) - ↑ 31.0 31.1 Hangaishi, Akira; et al. (2002-04-15). "Mutations of Chk2 in primary hematopoietic neoplasms". Blood. 99 (8): 3075–3077. doi:10.1182/blood.v99.8.3075. ISSN 0006-4971. PMID 11949635.
- ↑ 32.0 32.1 Camacho, Emma; et al. (2002-01-01). "ATM gene inactivation in mantle cell lymphoma mainly occurs by truncating mutations and missense mutations involving the phosphatidylinositol-3 kinase domain and is associated with increasing numbers of chromosomal imbalances". Blood. 99 (1): 238–244. doi:10.1182/blood.v99.1.238. ISSN 0006-4971. PMID 11756177.
- ↑ 33.0 33.1 Wang, Xiaogan; et al. (2021-01). "Germline variants of DNA repair genes in early onset mantle cell lymphoma". Oncogene. 40 (3): 551–563. doi:10.1038/s41388-020-01542-2. ISSN 1476-5594. PMID 33191405 Check
|pmid=value (help). Check date values in:|date=(help)
(use "Cite" icon at top of page)
Notes
*Primary authors will typically be those that initially create and complete the content of a page. If a subsequent user modifies the content and feels the effort put forth is of high enough significance to warrant listing in the authorship section, please contact the CCGA coordinators (contact information provided on the homepage). Additional global feedback or concerns are also welcome.
*Citation of this Page: “In situ mantle cell neoplasm”. Compendium of Cancer Genome Aberrations (CCGA), Cancer Genomics Consortium (CGC), updated 12/4/2023, https://ccga.io/index.php/HAEM5:In_situ_mantle_cell_neoplasm.