B-lymphoblastic leukaemia/lymphoma with BCR::ABL1-like features
Haematolymphoid Tumours (WHO Classification, 5th ed.)
| This page is under construction |
editContent Update To WHO 5th Edition Classification Is In Process; Content Below is Based on WHO 4th Edition ClassificationThis page was converted to the new template on 2023-12-07. The original page can be found at HAEM4:B-Lymphoblastic Leukemia/Lymphoma, BCR-ABL1-Like.
(General Instructions – The main focus of these pages is the clinically significant genetic alterations in each disease type. Use HUGO-approved gene names and symbols (italicized when appropriate), HGVS-based nomenclature for variants, as well as generic names of drugs and testing platforms or assays if applicable. Please complete tables whenever possible and do not delete them (add N/A if not applicable in the table and delete the examples); to add (or move) a row or column to a table, click within the table and select the > symbol that appears to be given options. Please do not delete or alter the section headings. The use of bullet points alongside short blocks of text rather than only large paragraphs is encouraged. Additional instructions below in italicized blue text should not be included in the final page content. Please also see Author_Instructions and FAQs as well as contact your Associate Editor or Technical Support)
Primary Author(s)*
Mark G. Evans, MD, University of California, Irvine
Fabiola Quintero-Rivera, MD, University of California, Irvine
Cancer Category / Type
Acute Lymphoblastic Leukemia
Cancer Sub-Classification / Subtype
B-lymphoblastic leukemia/lymphoma, BCR-ABL1-like
Definition / Description of Disease
In 2009, a high-risk subgroup of B-ALL was identified in children, adolescents, and young adults[1][2][3]. The genetic expression is similar to that of BCR-ABL1-positive cases, but without t(9;22)(q34.1;q11.2). Instead, BCR-ABL-1-like B-ALL is a genetically heterogenous disease, often with alterations activating cytokine receptors and tyrosine kinases. Several genetic expression profiles were initially utilized to recognize cases[1][2], however, different profiles did not always identify the same patients[4]. Although emerging data advocates the therapeutic use of tyrosine kinase or JAK inhibitors in this disease process, BCR-ABL-like B-ALL is often associated with very high rates of relapse and poor overall survival; thus proper diagnosis is essential[5].
Synonyms / Terminology
Ph-like B-lymphoblastic leukemia/lymphoma
Epidemiology / Prevalence
- Ph-like ALL comprises up to 15% of childhood B-ALL, and 20 to 25% in adolescents and young adults[6].
- The incidence in adult patients is controversial—from 13-17% to up to 33% in some reports[7][8][9].
- Higher rates of disease are observed in Hispanic and Native-American populations, and among children with Down syndrome[10].
Clinical Features
Put your text here and fill in the table (Instruction: Can include references in the table. Do not delete table.)
| Signs and Symptoms | EXAMPLE: Asymptomatic (incidental finding on complete blood counts)
EXAMPLE: B-symptoms (weight loss, fever, night sweats) EXAMPLE: Fatigue EXAMPLE: Lymphadenopathy (uncommon) |
| Laboratory Findings | EXAMPLE: Cytopenias
EXAMPLE: Lymphocytosis (low level) |
editv4:Clinical FeaturesThe content below was from the old template. Please incorporate above.The presenting symptoms are similar to those of other ALL patients, with the exception of potentially higher white blood cell counts[11].
Sites of Involvement
Bone marrow
Morphologic Features
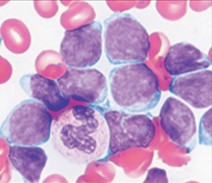
There are no morphological or cytochemical features that aid in the diagnosis. Blasts range from small to large and chromatin varying from immature to more mature, corresponding to French-American-British classification L1 or L2 subtype[12].
Immunophenotype
Put your text here and fill in the table (Instruction: Can include references in the table. Do not delete table.)
| Finding | Marker |
|---|---|
| Positive (universal) | EXAMPLE: CD1 |
| Positive (subset) | EXAMPLE: CD2 |
| Negative (universal) | EXAMPLE: CD3 |
| Negative (subset) | EXAMPLE: CD4 |
editv4:ImmunophenotypeThe content below was from the old template. Please incorporate above.Blasts are typically CD19, TdT, and CD10-positive. By flow cytometry, a subset of cases with CRLF2 translocations show very high levels of surface protein expression[11].
Chromosomal Rearrangements (Gene Fusions)
Put your text here and fill in the table
| Chromosomal Rearrangement | Genes in Fusion (5’ or 3’ Segments) | Pathogenic Derivative | Prevalence | Diagnostic Significance (Yes, No or Unknown) | Prognostic Significance (Yes, No or Unknown) | Therapeutic Significance (Yes, No or Unknown) | Notes |
|---|---|---|---|---|---|---|---|
| EXAMPLE: t(9;22)(q34;q11.2) | EXAMPLE: 3'ABL1 / 5'BCR | EXAMPLE: der(22) | EXAMPLE: 20% (COSMIC)
EXAMPLE: 30% (add reference) |
Yes | No | Yes | EXAMPLE:
The t(9;22) is diagnostic of CML in the appropriate morphology and clinical context (add reference). This fusion is responsive to targeted therapy such as Imatinib (Gleevec) (add reference). |
editv4:Chromosomal Rearrangements (Gene Fusions)The content below was from the old template. Please incorporate above.Tyrosine kinase-type translocations are common and involve ABL1 and other kinases (such as ABL2, EPOR, JAK2, PDGFRB, and CSF1R); more than 30 gene partners have been described. Frequently reported examples include IGH–EPOR of the t(14;19)(q32;p13)/ins(14;19)(q32;p13), EBF1–PDGFRB of the del(5)(q32q33.3), NUP214–ABL1 of the t(9;9)(q34;q34)/del(9)(q34q34), and ETV6–ABL1 of the t(9;12)(q34;p13). Other notable fusions are BCR–JAK2, PAX5–JAK2, STRN3–JAK2, RANBP2–ABL1, RCSD1–ABL1, and MEF2D–CSF1R[13].
editv4:Clinical Significance (Diagnosis, Prognosis and Therapeutic Implications).Please incorporate this section into the relevant tables found in:
- Chromosomal Rearrangements (Gene Fusions)
- Individual Region Genomic Gain/Loss/LOH
- Characteristic Chromosomal Patterns
- Gene Mutations (SNV/INDEL)
- Diagnosis: Definitive diagnosis is based on two major gene expression signatures (DCOG/Erasmus MC and COG/St. Jude).
- DCOG/Erasmus MC incorporates hierarchal clustering of microarrays using a 110-gene probe set; this genetic signature frequently detected deletions in IKZF1, dic(9;20), and iAMP21 in BCR-ABL1-like B-ALL[1].
- COG/St. Jude employs predictive analysis of microarrays using a 257-gene probe set; this genetic signature demonstrated primarily activating kinase or cytokine receptor signaling alterations, in addition to IKZF1 deletions[2].
- Prognosis: In both pediatric and adult populations, BCR-ABL1-like B-ALL is associated with high rates of relapse and poor prognosis.
- The median 5-year overall survival rates for children with BCR-ABL1-like B-ALL, adolescents, and young adults was 72.8%, 65.8%, and 25.8%, respectively[6].
- Median 5-year-overall survival in adults was 22%, versus 64% in comparable patients with non-BCR-ABL1, non-BCR-ABL1-like, and non-MLL translocation B-ALL[8].
- Therapeutic Implications: Due to the aggressive nature of the disease, patients are often treated with high-intensity chemotherapy regimens, such as hyper-CVAD or an augmented Berlin-Frankfurt-Münster regimen[9].
Individual Region Genomic Gain / Loss / LOH
Put your text here and fill in the table (Instructions: Includes aberrations not involving gene fusions. Can include references in the table. Can refer to CGC workgroup tables as linked on the homepage if applicable. Do not delete table.)
| Chr # | Gain / Loss / Amp / LOH | Minimal Region Genomic Coordinates [Genome Build] | Minimal Region Cytoband | Diagnostic Significance (Yes, No or Unknown) | Prognostic Significance (Yes, No or Unknown) | Therapeutic Significance (Yes, No or Unknown) | Notes |
|---|---|---|---|---|---|---|---|
| EXAMPLE:
7 |
EXAMPLE: Loss | EXAMPLE:
chr7:1- 159,335,973 [hg38] |
EXAMPLE:
chr7 |
Yes | Yes | No | EXAMPLE:
Presence of monosomy 7 (or 7q deletion) is sufficient for a diagnosis of AML with MDS-related changes when there is ≥20% blasts and no prior therapy (add reference). Monosomy 7/7q deletion is associated with a poor prognosis in AML (add reference). |
| EXAMPLE:
8 |
EXAMPLE: Gain | EXAMPLE:
chr8:1-145,138,636 [hg38] |
EXAMPLE:
chr8 |
No | No | No | EXAMPLE:
Common recurrent secondary finding for t(8;21) (add reference). |
editv4:Genomic Gain/Loss/LOHThe content below was from the old template. Please incorporate above.Monoallelic (often partial) deletion of the IKAROS transcription factor, encoded by IKZF1, is one of the most frequently observed genetic abnormalities in BCR-ABL1-like B-ALL, although this finding is not specific and not included in the definition[4].
Characteristic Chromosomal Patterns
Put your text here (EXAMPLE PATTERNS: hyperdiploid; gain of odd number chromosomes including typically chromosome 1, 3, 5, 7, 11, and 17; co-deletion of 1p and 19q; complex karyotypes without characteristic genetic findings; chromothripsis. Do not delete table.)
| Chromosomal Pattern | Diagnostic Significance (Yes, No or Unknown) | Prognostic Significance (Yes, No or Unknown) | Therapeutic Significance (Yes, No or Unknown) | Notes |
|---|---|---|---|---|
| EXAMPLE:
Co-deletion of 1p and 18q |
Yes | No | No | EXAMPLE:
See chromosomal rearrangements table as this pattern is due to an unbalanced derivative translocation associated with oligodendroglioma (add reference). |
editv4:Characteristic Chromosomal Aberrations / PatternsThe content below was from the old template. Please incorporate above.Approximately half of cases demonstrate rearrangements resulting in overexpression of CRLF2[12]. These rearrangements are the result of either translocation of immunoglobin heavy chain enhance locus into CRLF2 (IGH-CRLF2—more commonly seen in adults) or through a cryptic deletion on chromosome X/Y involving the PAR1 psuedoautosomal region, resulting in fusion of CRLF2 to P2RY8 (more commonly seen in children). Very rare alternative translocations involving CRLF2 have also been observed.
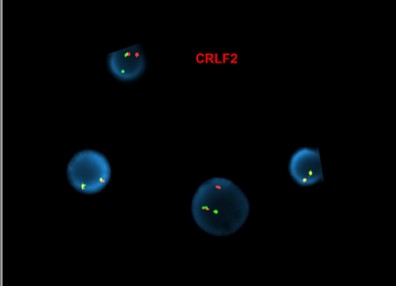
[Abnormal FISH results in interphase nuclei from a bone marrow sample using the CRLF2 dual-color, break-apart (Cytocell) and IGH dual-color, break-apart probes, reflective of IGH-CRLF2 fusion]
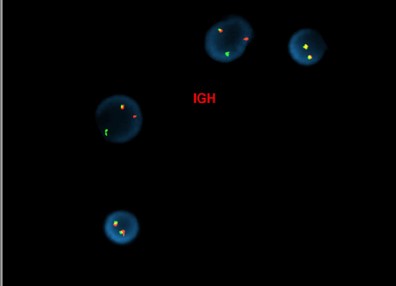
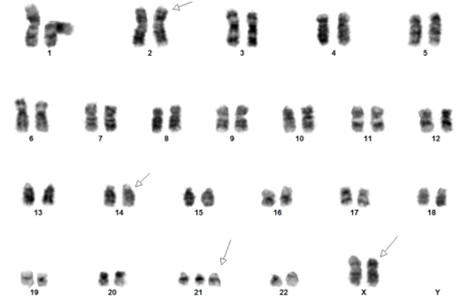
[Concurrent abnormal karyotype with trisomy 21 and a translocation involving chromosomes X, 14, and 2 in 9 of 13 cells available for analysis. Metaphase FISH with the IGH break-apart probe (Vysis) confirms the presence of 5’ IGH (green signal) on the abnormal chromosome Xp33.1 (CRLF2 locus), highly suggestive on an IGH-CRLF2 fusion rearrangement.
47,XX,+21c[4]/47,idem,der(X)t(X;14)(p33.1;q32),der(2)t(2;14)(p11.2;q11.2)t(X;14),der(14)t(2;14)[5]/46,XX[4].ish der(X)(5’IGH+),der(2)(3’IGH+)]
(Images courtesy of Fabiola Quintero-Rivera, MD)
Gene Mutations (SNV / INDEL)
Put your text here and fill in the table (Instructions: This table is not meant to be an exhaustive list; please include only genes/alterations that are recurrent and common as well as either disease defining and/or clinically significant. Can include references in the table. For clinical significance, denote associations with FDA-approved therapy (not an extensive list of applicable drugs) and NCCN or other national guidelines if applicable. Can also refer to CGC workgroup tables as linked on the homepage if applicable as well as any high impact papers or reviews of gene mutations in this entity. Do not delete table.)
| Gene; Genetic Alteration | Presumed Mechanism (Tumor Suppressor Gene [TSG] / Oncogene / Other) | Prevalence (COSMIC / TCGA / Other) | Concomitant Mutations | Mutually Exclusive Mutations | Diagnostic Significance (Yes, No or Unknown) | Prognostic Significance (Yes, No or Unknown) | Therapeutic Significance (Yes, No or Unknown) | Notes |
|---|---|---|---|---|---|---|---|---|
| EXAMPLE: TP53; Variable LOF mutations
EXAMPLE: EGFR; Exon 20 mutations EXAMPLE: BRAF; Activating mutations |
EXAMPLE: TSG | EXAMPLE: 20% (COSMIC)
EXAMPLE: 30% (add Reference) |
EXAMPLE: IDH1 R123H | EXAMPLE: EGFR amplification | EXAMPLE: Excludes hairy cell leukemia (HCL) (add reference).
|
Note: A more extensive list of mutations can be found in cBioportal (https://www.cbioportal.org/), COSMIC (https://cancer.sanger.ac.uk/cosmic), ICGC (https://dcc.icgc.org/) and/or other databases. When applicable, gene-specific pages within the CCGA site directly link to pertinent external content.
editv4:Gene Mutations (SNV/INDEL)The content below was from the old template. Please incorporate above.In addition to gene translocations, gain-of-function mutations in CRLF2 itself or in its partner gene, IL7RA, have been seen[16]. Alternative alterations activating kinase signaling occur, including activating mutations of FLT3, as well as focal deletions of SH2B3 (also known as LNK)[17].
Herold et al. in 2017 reported a wide variety of molecular alterations in BCR-ABL1-like B-ALL, which was shown to have statistically significant associations with alterations of IKZF1, CRLF2, JAK2, BTG1, and high CRLF2 expression[8].
Epigenomic Alterations
Not applicable
Genes and Main Pathways Involved
Put your text here and fill in the table (Instructions: Can include references in the table. Do not delete table.)
| Gene; Genetic Alteration | Pathway | Pathophysiologic Outcome |
|---|---|---|
| EXAMPLE: BRAF and MAP2K1; Activating mutations | EXAMPLE: MAPK signaling | EXAMPLE: Increased cell growth and proliferation |
| EXAMPLE: CDKN2A; Inactivating mutations | EXAMPLE: Cell cycle regulation | EXAMPLE: Unregulated cell division |
| EXAMPLE: KMT2C and ARID1A; Inactivating mutations | EXAMPLE: Histone modification, chromatin remodeling | EXAMPLE: Abnormal gene expression program |
editv4:Genes and Main Pathways InvolvedThe content below was from the old template. Please incorporate above.
- IKAROS transcription factor: Deletion of IKZF1 results in activation of EBF1, MSH2, and MCL1, leading to B-cell leukemogenesis[18].
- CRLF2 overexpression: CRFL2 and its cofactor IL7RA form a receptor for thymic stromal-derived lymphopoietin that activates the JAK2-signal transducer and upregulates the transcription 5 pathway[16].
- Dysregulation of several tyrosine kinase signaling pathways (involving ABL1, ABL2, PDGFRB, CSF1, etc.) results in B-cell progenitor proliferation.
Genetic Diagnostic Testing Methods
- Flow cytometry for CRLF2 has been shown in some studies to be 100% concordant with FISH results[12].
- Next-generation sequencing is helpful for detecting copy number changes, single nucleotide variants, and gene fusions involving CRLF2, ABL1, ABL2, JAK2, etc.
- Gene expression profile algorithms, incorporating prediction analysis or hierarchical clustering of microarrays, provide the definitive diagnosis of BCR-ABL1-like B-ALL.
Familial Forms
Families with certain inherited variants of GATA3 (often seen in Native-American populations) are at increased risk of BCR-ABL1-like B-ALL[19].
Additional Information
Put your text here
Links
Pre-B ALL B-lymphoblastic leukemia/lymphoma with BCR-ABL1-like/Ph-like in Pathology Outlines (http://www.pathologyoutlines.com/topic/leukemiaprebbcrabl1like.html)
References
(use the "Cite" icon at the top of the page) (Instructions: Add each reference into the text above by clicking on where you want to insert the reference, selecting the “Cite” icon at the top of the page, and using the “Automatic” tab option to search such as by PMID to select the reference to insert. The reference list in this section will be automatically generated and sorted. If a PMID is not available, such as for a book, please use the “Cite” icon, select “Manual” and then “Basic Form”, and include the entire reference.)
- ↑ 1.0 1.1 1.2 Den Boer, Monique L.; et al. (2009). "A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study". The Lancet. Oncology. 10 (2): 125–134. doi:10.1016/S1470-2045(08)70339-5. ISSN 1474-5488. PMC 2707020. PMID 19138562.
- ↑ 2.0 2.1 2.2 Mullighan, Charles G.; et al. (2009). "Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia". The New England Journal of Medicine. 360 (5): 470–480. doi:10.1056/NEJMoa0808253. ISSN 1533-4406. PMC 2674612. PMID 19129520.
- ↑ Roberts, Kathryn G.; et al. (2012). "Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia". Cancer Cell. 22 (2): 153–166. doi:10.1016/j.ccr.2012.06.005. ISSN 1878-3686. PMC 3422513. PMID 22897847.
- ↑ 4.0 4.1 Boer, Judith M.; et al. (2015). "BCR-ABL1-like cases in pediatric acute lymphoblastic leukemia: a comparison between DCOG/Erasmus MC and COG/St. Jude signatures". Haematologica. 100 (9): e354–357. doi:10.3324/haematol.2015.124941. ISSN 1592-8721. PMC 4800707. PMID 26045294.
- ↑ Roberts, Kathryn G.; et al. (2018). "Genomic and outcome analyses of Ph-like ALL in NCI standard-risk patients: a report from the Children's Oncology Group". Blood. 132 (8): 815–824. doi:10.1182/blood-2018-04-841676. ISSN 1528-0020. PMC 6107876. PMID 29997224.
- ↑ 6.0 6.1 6.2 Roberts, Kathryn G.; et al. (2014). "Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia". The New England Journal of Medicine. 371 (11): 1005–1015. doi:10.1056/NEJMoa1403088. ISSN 1533-4406. PMC 4191900. PMID 25207766.
- ↑ Boer, Judith M.; et al. (2015). "Expression profiling of adult acute lymphoblastic leukemia identifies a BCR-ABL1-like subgroup characterized by high non-response and relapse rates". Haematologica. 100 (7): e261–264. doi:10.3324/haematol.2014.117424. ISSN 1592-8721. PMC 4486237. PMID 25769542.
- ↑ 8.0 8.1 8.2 Herold, Tobias; et al. (2017). "Adults with Philadelphia chromosome-like acute lymphoblastic leukemia frequently have IGH-CRLF2 and JAK2 mutations, persistence of minimal residual disease and poor prognosis". Haematologica. 102 (1): 130–138. doi:10.3324/haematol.2015.136366. ISSN 1592-8721. PMC 5210243. PMID 27561722.
- ↑ 9.0 9.1 Jain, Nitin; et al. (2017). "Ph-like acute lymphoblastic leukemia: a high-risk subtype in adults". Blood. 129 (5): 572–581. doi:10.1182/blood-2016-07-726588. ISSN 1528-0020. PMC 5290985. PMID 27919910.
- ↑ Harvey, Richard C.; et al. (2010). "Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia". Blood. 115 (26): 5312–5321. doi:10.1182/blood-2009-09-245944. ISSN 1528-0020. PMC 2902132. PMID 20139093.
- ↑ 11.0 11.1 Borowitz MJ, et al., (2017). B-lymphoblastic leukaemia/lymphoma with recurrent genetic abnormalities, in World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues, Revised 4th edition. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Arber DA, Hasserjian RP, Le Beau MM, Orazi A, and Siebert R, Editors. IARC Press: Lyon, France, p208.
- ↑ 12.0 12.1 12.2 Konoplev, Sergej; et al. (2017). "CRLF2-Positive B-Cell Acute Lymphoblastic Leukemia in Adult Patients: A Single-Institution Experience". American Journal of Clinical Pathology. 147 (4): 357–363. doi:10.1093/ajcp/aqx005. ISSN 1943-7722. PMID 28340183.
- ↑ Heim S & Mitelman F. Cancer Cytogenetics: Chromosomal and Molecular Genetic Aberrations of Tumor Cells. John Wiley & Sons, Incorporated: Chichester, United Kingdom. 2015.
- ↑ Tasian, Sarah K.; et al. (2012). "Aberrant STAT5 and PI3K/mTOR pathway signaling occurs in human CRLF2-rearranged B-precursor acute lymphoblastic leukemia". Blood. 120 (4): 833–842. doi:10.1182/blood-2011-12-389932. ISSN 1528-0020. PMC 3412346. PMID 22685175.
- ↑ Iacobucci, Ilaria; et al. (2016). "Truncating Erythropoietin Receptor Rearrangements in Acute Lymphoblastic Leukemia". Cancer Cell. 29 (2): 186–200. doi:10.1016/j.ccell.2015.12.013. ISSN 1878-3686. PMC 4750652. PMID 26859458.
- ↑ 16.0 16.1 Quesada A, Reynolds M, Jorgensen JL, et al. Cytokine receptor-like factor 2 (CRLF2) expression in precursor B-lymphoblastic leukemia. International Clinical Cytometry Society e-Newsletter. 2014;5(1).
- ↑ Tosi S & Reid AG. The Genetic Basis of Haematological Cancers. John Wiley & Sons, Incorporated: Chichester, United Kingdom: 2016.
- ↑ van der Veer, Arian; et al. (2013). "Independent prognostic value of BCR-ABL1-like signature and IKZF1 deletion, but not high CRLF2 expression, in children with B-cell precursor ALL". Blood. 122 (15): 2622–2629. doi:10.1182/blood-2012-10-462358. ISSN 1528-0020. PMC 3795461. PMID 23974192.
- ↑ Perez-Andreu, Virginia; et al. (2013). "Inherited GATA3 variants are associated with Ph-like childhood acute lymphoblastic leukemia and risk of relapse". Nature Genetics. 45 (12): 1494–1498. doi:10.1038/ng.2803. ISSN 1546-1718. PMC 4039076. PMID 24141364.
Notes
*Primary authors will typically be those that initially create and complete the content of a page. If a subsequent user modifies the content and feels the effort put forth is of high enough significance to warrant listing in the authorship section, please contact the CCGA coordinators (contact information provided on the homepage). Additional global feedback or concerns are also welcome. *Citation of this Page: “B-lymphoblastic leukaemia/lymphoma with BCR::ABL1-like features”. Compendium of Cancer Genome Aberrations (CCGA), Cancer Genomics Consortium (CGC), updated 09/6/2024, https://ccga.io/index.php/HAEM5:B-lymphoblastic_leukaemia/lymphoma_with_BCR::ABL1-like_features.