Difference between revisions of "HAEM4:Myelodysplastic Syndrome (MDS) with Isolated del(5q)"
Bailey.Glen (talk | contribs) (Created page with " ==Primary Author(s)*== Xiaolin Hu, Ph.D; Teresa Smolarek, Ph.D, FACMG __TOC__ del(5)(q15q33)|322x322px ==Cancer Category/Type== Myelodysplast...") |
Bailey.Glen (talk | contribs) |
||
| Line 1: | Line 1: | ||
| + | {{DISPLAYTITLE:Myelodysplastic Syndrome (MDS) with Isolated del(5q)}} | ||
| + | |||
| + | <blockquote class='blockedit'>{{Box-round|title=PREVIOUS EDITION|This page from the 4th edition of Haematolymphoid Tumours is being updated. See 5th edition [[HAEM5:Table_of_Contents|Table of Contents]]. | ||
| + | }}</blockquote> | ||
==Primary Author(s)*== | ==Primary Author(s)*== | ||
| Line 185: | Line 189: | ||
==Notes== | ==Notes== | ||
<nowiki>*</nowiki>Primary authors will typically be those that initially create and complete the content of a page. If a subsequent user modifies the content and feels the effort put forth is of high enough significance to warrant listing in the authorship section, please contact the CCGA coordinators (contact information provided on the homepage). Additional global feedback or concerns are also welcome. | <nowiki>*</nowiki>Primary authors will typically be those that initially create and complete the content of a page. If a subsequent user modifies the content and feels the effort put forth is of high enough significance to warrant listing in the authorship section, please contact the CCGA coordinators (contact information provided on the homepage). Additional global feedback or concerns are also welcome. | ||
| + | [[Category:HAEM4]] [[Category:DISEASE]] | ||
Revision as of 13:43, 3 November 2023
editPREVIOUS EDITIONThis page from the 4th edition of Haematolymphoid Tumours is being updated. See 5th edition Table of Contents.
Primary Author(s)*
Xiaolin Hu, Ph.D; Teresa Smolarek, Ph.D, FACMG
Cancer Category/Type
Myelodysplastic syndromes (MDS)
Cancer Sub-Classification / Subtype
Myelodysplastic syndromes with isolated del(5q)
Definition / Description of Disease
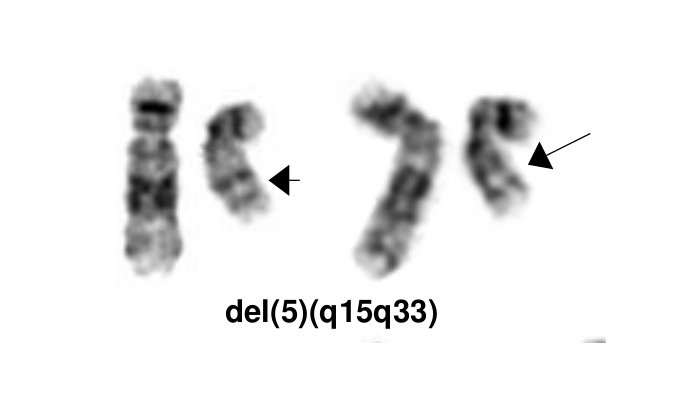
MDS with isolated del(5q) is a type of MDS with defining cytogenetic abnormality of del(5q). The World Health Organization named it as isolated del 5q, but occasionally the deletion can occur with an additional cytogenetic abnormality other than monosomy 7 or del(7q). The 5q- syndrome was first described by Van den Berghe et al as a distinct type of MDS featured with macrocytic anemia, hypolobulated megakaryocytes, a normal or increased platelet count [1]. Deletion of 5q is the most common recurrent cytogenetic abnormality in myeloid neoplasm and it was commonly seen in 10-15% of patients with MDS[2]. A ~1.5 Mb common deleted region (CDR) at 5q32-q33 was identified in 5q- syndrome patients and was associated with good prognosis [3]. This disease has a good respond to lenalidomide treatment (See Clinical Significance).
Synonyms / Terminology
Myelodysplastic syndromes with 5q deletion; 5q minus syndrome
Epidemiology / Prevalence
- Median age 67 years [4]
- Women versus male about 2:1
Clinical Features
- Macrocytic anemia most common, usually severe and can be transfusion dependent.
- Thrombocytosis can be seen in 1/3 to 1/2 of cases, whereas thrombocytopenia or neutropenia are uncommon.
- Pancytopenia is rare. If pancytopenia with isolated 5q-, MDS with unclassifiable should be considered. [WHO]
- Good prognosis and low risk to progress to AML.
Sites of Involvement
Blood or bone marrow
Morphologic Features
On bone marrow examination, cellularity is usually normal or hypercellular. Erythroid hypoplasia may be seen. Blasts cells are < 5% in the bone marrow and < 1% in the peripheral blood. The most characteristic feature of isolated 5q- is the presence of micromegakaryocytes with hypolobated nuclei [insert pic micromegakaryocyte]. The erythroid dysplasia may be seen but not predominant. Granulocytic dysplasia is uncommon.
Hypolobated micromegakaryocytes are also seen in CML, which is characterized by leukocytosis, basophilia, and myeloid hyperplasia with leftward shift. Platelet count increase can be seen in essential thrombocythemia (ET), but ET has large megakaryocytes with hyperlobulated nuclei rather than microkaryocytes with hypolobulation in MDS with isolated del(5q).
Immunophenotype
There is no distinct immunophenotypic profile specific for myelodysplastic syndrome (MDS) with isolated del(5q). Currently, morphologic evaluation remains the gold standard in diagnosis of MDS. Immunophenotyping provides supportive evidence to clarify the blasts nature and percentage [5].
Chromosomal Rearrangements (Gene Fusions)
NA
| Chromosomal Rearrangement | Genes in Fusion (5’ or 3’ Segments) | Pathogenic Derivative | Prevalence |
|---|---|---|---|
| EXAMPLE t(9;22)(q34;q11.2) | EXAMPLE 3'ABL1 / 5'BCR | EXAMPLE der(22) | EXAMPLE 5% |
| EXAMPLE t(8;21)(q22;q22) | EXAMPLE 5'RUNX1 / 3'RUNXT1 | EXAMPLE der(8) | EXAMPLE 5% |
Characteristic Chromosomal Aberrations / Patterns
The most characteristic cytogenetic abnormality is an interstitial deletion on the long arm of chromosome 5 or del(5q)[1] The break point is not fixed but the region between bands q31 and q33 is generally deleted. Several candidates genes were thought to contribute to the haploinsufficiency effect of the deleted region, including RPS14 [6], CSNK1A1 [7], miR-145 and miR-146a [8]. Identification of del(5q) has clinical significance because patients with this cytogenetic abnormality have a good prognosis and they respond well to lenalidomide treatment.
Genomic Gain/Loss/LOH
| Chromosome Number | Gain/Loss/Amp/LOH | Region |
|---|---|---|
| EXAMPLE 8 | EXAMPLE Gain | EXAMPLE chr8:0-1000000 |
| EXAMPLE 7 | EXAMPLE Loss | EXAMPLE chr7:0-1000000 |
Gene Mutations (SNV/INDEL)
Somatic mutations in JAK2 and MPL have been reported in a small subset patients with isolated del(5q), but these mutations seem not confer diagnostic or prognostic value [9]. A subset of cases could have SF3B1 mutations, which needs to be differentiated with MDS-RS.
| Gene | Mutation | Oncogene/Tumor Suppressor/Other | Presumed Mechanism (LOF/GOF/Other; Driver/Passenger) | Prevalence (COSMIC/TCGA/Other) |
|---|---|---|---|---|
| EXAMPLE TP53 | EXAMPLE R273H | EXAMPLE Tumor Suppressor | EXAMPLE LOF | EXAMPLE 20% |
Other Mutations
| Type | Gene/Region/Other |
|---|---|
| Concomitant Mutations | JAK2 V617F, MPL W515L |
| Secondary Mutations | |
| Mutually Exclusive |
Epigenomics (Methylation)
Genes involved in epigenetic regulation are frequently mutated in MDS such as TET2, DNMT3A, IDH1, IDH2, AXSL1, and EZH2 [10]. These genes play a role in DNA methylation and chromatin modification as well as regulating gene expression.
Genes and Main Pathways Involved
Several candidate genes in the common deleted region of 5q have been reported to contribute to the molecular pathogenesis.
RPS14: Encodes for ribosomal subunit of 40S. Inactivation of RPS14 results in ineffective erythroid differentiation and increased apoptosis in a p53 dependent way [11].
CSNK1A1: Encodes for CK1α, a serine/threonine protein kinase activity. Mutations in CSNK1A1 lead to clonal expansion of hematopoietic stem cells through activation of β-catenin [7].
miR-145 and miR146α: These two micro RNA genes are thought to be associated with thrombocytosis and hypolobulated megakaryocytes [12].
Diagnostic Testing Methods
- Bone marrow morphology and peripheral blood test are standard in diagnosis of MDS.
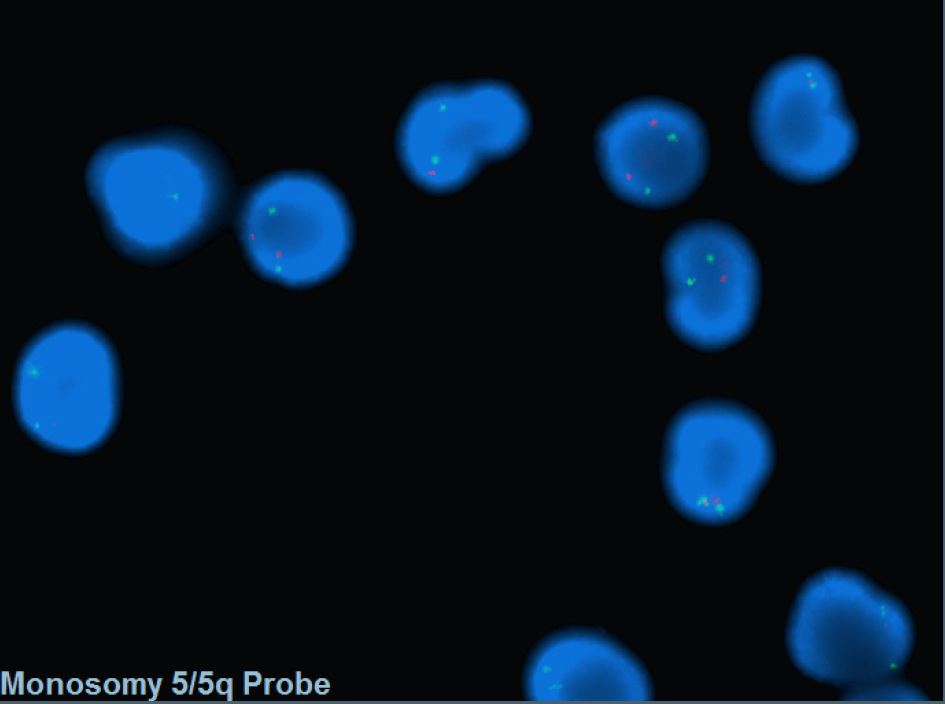
- FISH probes targeting the CDR are commonly incorporated into MDS panel to assess -5/5q-.
- Conventional cytogenetics are also helpful in diagnosis.
Clinical Significance (Diagnosis, Prognosis and Therapeutic Implications)
Diagnosis:
- Cytopenia in 1 to 2 lineages. Cytopenias are defined as hemoglobin concentration < 10 g/dL, platelet count < 100 x 109/L and absolute neutrophil count < 1.8 x 109/L; mild degree of anemia (hemoglobin < 13 g/dL in men or < 12 g/dL in women) or thrombocytopenia (platelets < 150 x109/L) are allowed if defining cytogenetic abnormality is present. Thrombocytosis (platelet count ≥ 450 x 109/L) is allowed in MDS with isolated del(5q). PB monocytes must be < 1 x109/L.
- Dysplasia in 1 to 3 lineages. Micromegakaryocytes with hypolobation are characteristic dysplasia. Ring sideroblasts are not common.
- Blasts percentage: BM <5%, PB <1%, no Auer rods.
- Interstitial deletion of 5q alone or with an additional abnormality other than loss of chromosome 7 or deletion of 7q.
- Chromosome 7 loss or del(7q), more than one additional cytogenetic abnormality, excess blasts need to be excluded from the diagnosis.
Prognosis:
- According to IPSS‐R good‐risk group with low rates of leukemic transformation [13].
- The median survival of MDS with isolated del(5q) is 66 to 145 months.
- About less than 10% of cases progress to acute myeloid leukemia [14].
- Mutations of TP53 may function as an independent prognostic factor in associated with leukemic progression and resistant to lenalidomide[15][16].
Therapeutic Implications:
Lenalidomide is an analogue of thalidomide which functions as an immunomodulatory agent. Treatment with lenalidomide has shown great potential in reducing the abnormal clone as well as transfusion dependency irrespective of cytogenetic complexity [17][18]. FDA approved Lenalidomide on December 2005 for treating MDS with del(5q) with or without additional cytogenetic abnormalities.
Familial Forms
Put your text here
Other Information
Put your text here
Links
Put your links here (use link icon at top of page)
References
- ↑ 1.0 1.1 Van Den Berghe, Herman; et al. (1974). "Distinct haematological disorder with deletion of long arm of No. 5 chromosome". Nature. 251 (5474): 437–438. doi:10.1038/251437a0. ISSN 0028-0836.
- ↑ Hosono, Naoko; et al. (2017). "Recurrent genetic defects on chromosome 5q in myeloid neoplasms". Oncotarget. 8 (4): 6483–6495. doi:10.18632/oncotarget.14130. ISSN 1949-2553. PMC 5351647. PMID 28031539.CS1 maint: PMC format (link)
- ↑ Boultwood, J.; et al. (1994). "Molecular Mapping of Uncharacteristically Small 5q Deletions in Two Patients with the 5q- Syndrome: Delineation of the Critical Region on 5q and Identification of a 5q- Breakpoint". Genomics. 19 (3): 425–432. doi:10.1006/geno.1994.1090.
- ↑ Giagounidis, A.A.N.; et al. (2004). "Hematological Malignancies". Hematology. 9 (4): 271–277. doi:10.1080/10245330410001723824. ISSN 1607-8454.
- ↑ Zini, Gina (2017). "Diagnostics and Prognostication of Myelodysplastic Syndromes". Annals of Laboratory Medicine. 37 (6): 465. doi:10.3343/alm.2017.37.6.465. ISSN 2234-3806. PMC 5587818. PMID 28840983.CS1 maint: PMC format (link)
- ↑ Ebert, Benjamin L.; et al. (2008). "Identification of RPS14 as a 5q- syndrome gene by RNA interference screen". Nature. 451 (7176): 335–339. doi:10.1038/nature06494. ISSN 0028-0836. PMC 3771855. PMID 18202658.CS1 maint: PMC format (link)
- ↑ 7.0 7.1 Schneider, Rebekka K.; et al. (2014). "Role of Casein Kinase 1A1 in the Biology and Targeted Therapy of del(5q) MDS". Cancer Cell. 26 (4): 509–520. doi:10.1016/j.ccr.2014.08.001. PMC 4199102. PMID 25242043.CS1 maint: PMC format (link)
- ↑ Starczynowski, Daniel T; et al. (2010). "Identification of miR-145 and miR-146a as mediators of the 5q– syndrome phenotype". Nature Medicine. 16 (1): 49–58. doi:10.1038/nm.2054. ISSN 1078-8956.
- ↑ Patnaik, M M; et al. (2010). "WHO-defined 'myelodysplastic syndrome with isolated del(5q)' in 88 consecutive patients: survival data, leukemic transformation rates and prevalence of JAK2, MPL and IDH mutations". Leukemia. 24 (7): 1283–1289. doi:10.1038/leu.2010.105. ISSN 0887-6924. PMC 3035970. PMID 20485371.CS1 maint: PMC format (link)
- ↑ Heuser, Michael; et al. (2018). "Epigenetics in myelodysplastic syndromes". Seminars in Cancer Biology. 51: 170–179. doi:10.1016/j.semcancer.2017.07.009.
- ↑ Barlow, Jillian L; et al. (2010). "A p53-dependent mechanism underlies macrocytic anemia in a mouse model of human 5q– syndrome". Nature Medicine. 16 (1): 59–66. doi:10.1038/nm.2063. ISSN 1078-8956. PMC 2803774. PMID 19966810.CS1 maint: PMC format (link)
- ↑ Starczynowski, Daniel T; et al. (2010). "Identification of miR-145 and miR-146a as mediators of the 5q– syndrome phenotype". Nature Medicine. 16 (1): 49–58. doi:10.1038/nm.2054. ISSN 1078-8956.
- ↑ Mallo, M; et al. (2011). "Impact of adjunct cytogenetic abnormalities for prognostic stratification in patients with myelodysplastic syndrome and deletion 5q". Leukemia. 25 (1): 110–120. doi:10.1038/leu.2010.231. ISSN 0887-6924.
- ↑ Boultwood, Jacqueline; et al. (2010). "Advances in the 5q− syndrome". Blood. 116 (26): 5803–5811. doi:10.1182/blood-2010-04-273771. ISSN 0006-4971.
- ↑ Jädersten, Martin; et al. (2011). "TP53 Mutations in Low-Risk Myelodysplastic Syndromes With del(5q) Predict Disease Progression". Journal of Clinical Oncology. 29 (15): 1971–1979. doi:10.1200/JCO.2010.31.8576. ISSN 0732-183X.
- ↑ Kulasekararaj, Austin G.; et al. (2013). "TP53 mutations in myelodysplastic syndrome are strongly correlated with aberrations of chromosome 5, and correlate with adverse prognosis". British Journal of Haematology. 160 (5): 660–672. doi:10.1111/bjh.12203.
- ↑ Giagounidis, A. A. N.; et al. (2005). "Prognosis of patients with del(5q) MDS and complex karyotype and the possible role of lenalidomide in this patient subgroup". Annals of Hematology. 84 (9): 569–571. doi:10.1007/s00277-005-1054-0. ISSN 0939-5555.
- ↑ List, Alan; et al. (2006). "Lenalidomide in the Myelodysplastic Syndrome with Chromosome 5q Deletion". New England Journal of Medicine. 355 (14): 1456–1465. doi:10.1056/NEJMoa061292. ISSN 0028-4793.
1. Hasserjian RP, et al., (2017). Myelodysplastic syndrome with isolated del(5q), in World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues, Revised 4th edition. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Arber DA, Hasserjian RP, Le Beau MM, Orazi A, and Siebert R, Editors. IARC Press: Lyon, France, p115-116.
3. Pedersen B, Jensen IM. (1991). Clinical and prognostic implications of chromosome 5q deletions: 96 high resolution studied patients. Leukemia 5(7):566-573, PMID 2072742.
4. Rubin CM, et al., (1991). Therapy-related myelodysplastic syndrome and acute myeloid leukemia in children: correlation between chromosomal abnormalities and prior therapy. Blood 78(11):2982-2988, PMID 1954385.
5. Neuman WL, et al., (1992). Chromosomal loss and deletion are the most common mechanisms for loss of heterozygosity from chromosomes 5 and 7 in malignant myeloid disorders. Blood 79(6):1501-1510, PMID 1347709.
6. Baranger L, et al., (1994). Translocation t(5;12)(q31-q33;p12-p13): a non-random translocation associated with a myeloid disorder with eosinophilia. Br J Haematol 88(2):343-347, PMID 7803280.
7. Boultwood J, et al., (1994). The 5q-syndrome. Blood 84(10):3253-3260, PMID 7949083.
8. Boultwood J and Fidler C. Chromosomal deletions in myelodysplasia. Leuk Lymphoma 17(1-2):71-78, PMID 7773164.
9. Van den Berghe H and Michaux L. (1997). 5q-, twenty-five years later: a synopsis. Cancer Genet Cytogenet 94(1):1-7, PMID 9078284.
10. Giagounidis AA, et al., (2004). The 5q- syndrome. Hematology 9(4):271-277, PMID 15621734.
11. Nishino HT and Chang CC. (2005). Myelodysplastic syndromes: clinicopathologic features, pathobiology, and molecular pathogenesis. Arch Pathol Lab Med 129(10):1299-1310, PMID 16196520.
12. Bernasconi P, et al., (2006). Clinical relevance of cytogenetics in myelodysplastic syndromes. Ann N Y Acad Sci 1089:395-410, PMID 17261783.
13. Cherian S and Bagg A. (2006). The genetics of the myelodysplastic syndromes: classical cytogenetics and recent molecular insights. Hematology 11(1):1-13, PMID 16522543.
14. Armand P, et al., (2007). Impact of cytogenetics on outcome of de novo and therapy-related AML and MDS after allogeneic transplantation. Biol Blood Marrow Transplant 13(6):655-664, PMID 17531775.
15. Haase D. (2008). Cytogenetic features in myelodysplastic syndromes. Ann Hematol 87(7):515-526, PMID 18414863.
16. Kelaidi C., et al., (2008). The role of lenalidomide in the management of myelodysplasia with del 5q. Br J Haematol 140(3):267-278, PMID 18217896.
Notes
*Primary authors will typically be those that initially create and complete the content of a page. If a subsequent user modifies the content and feels the effort put forth is of high enough significance to warrant listing in the authorship section, please contact the CCGA coordinators (contact information provided on the homepage). Additional global feedback or concerns are also welcome.