Difference between revisions of "HAEM4:Chronic Myeloid Leukemia (CML), BCR-ABL1 Positive"
Bailey.Glen (talk | contribs) (Created page with "==Primary Author(s)*== Jack Reid, MD (University of California, Irvine) Mark Evans, MD (University of California, Irvine) Fabiola Quintero-Rivera, MD (University of Califor...") |
Bailey.Glen (talk | contribs) |
||
| Line 1: | Line 1: | ||
| + | {{DISPLAYTITLE:Chronic Myeloid Leukemia (CML), BCR-ABL1 Positive}} | ||
| + | |||
| + | |||
| + | <blockquote class='blockedit'>{{Box-round|title=PREVIOUS EDITION|This page from the 4th edition of Haematolymphoid Tumours is being updated. See 5th edition [[HAEM5:Table_of_Contents|Table of Contents]]. | ||
| + | }}</blockquote> | ||
==Primary Author(s)*== | ==Primary Author(s)*== | ||
| Line 121: | Line 126: | ||
==Notes== | ==Notes== | ||
<nowiki>*</nowiki>Primary authors will typically be those that initially create and complete the content of a page. If a subsequent user modifies the content and feels the effort put forth is of high enough significance to warrant listing in the authorship section, please contact the CCGA coordinators (contact information provided on the homepage). Additional global feedback or concerns are also welcome. | <nowiki>*</nowiki>Primary authors will typically be those that initially create and complete the content of a page. If a subsequent user modifies the content and feels the effort put forth is of high enough significance to warrant listing in the authorship section, please contact the CCGA coordinators (contact information provided on the homepage). Additional global feedback or concerns are also welcome. | ||
| − | + | [[Category:HAEM4]] [[Category:DISEASE]] | |
| − | [[Category: | ||
| − | [[Category: | ||
| − | |||
| − | |||
| − | |||
| − | |||
Latest revision as of 13:40, 3 November 2023
editPREVIOUS EDITIONThis page from the 4th edition of Haematolymphoid Tumours is being updated. See 5th edition Table of Contents.
Primary Author(s)*
Jack Reid, MD (University of California, Irvine)
Mark Evans, MD (University of California, Irvine)
Fabiola Quintero-Rivera, MD (University of California, Irvine)
Cancer Category/Type
Myeloproliferative neoplasm
Cancer Sub-Classification / Subtype
Not applicable.
Definition / Description of Disease
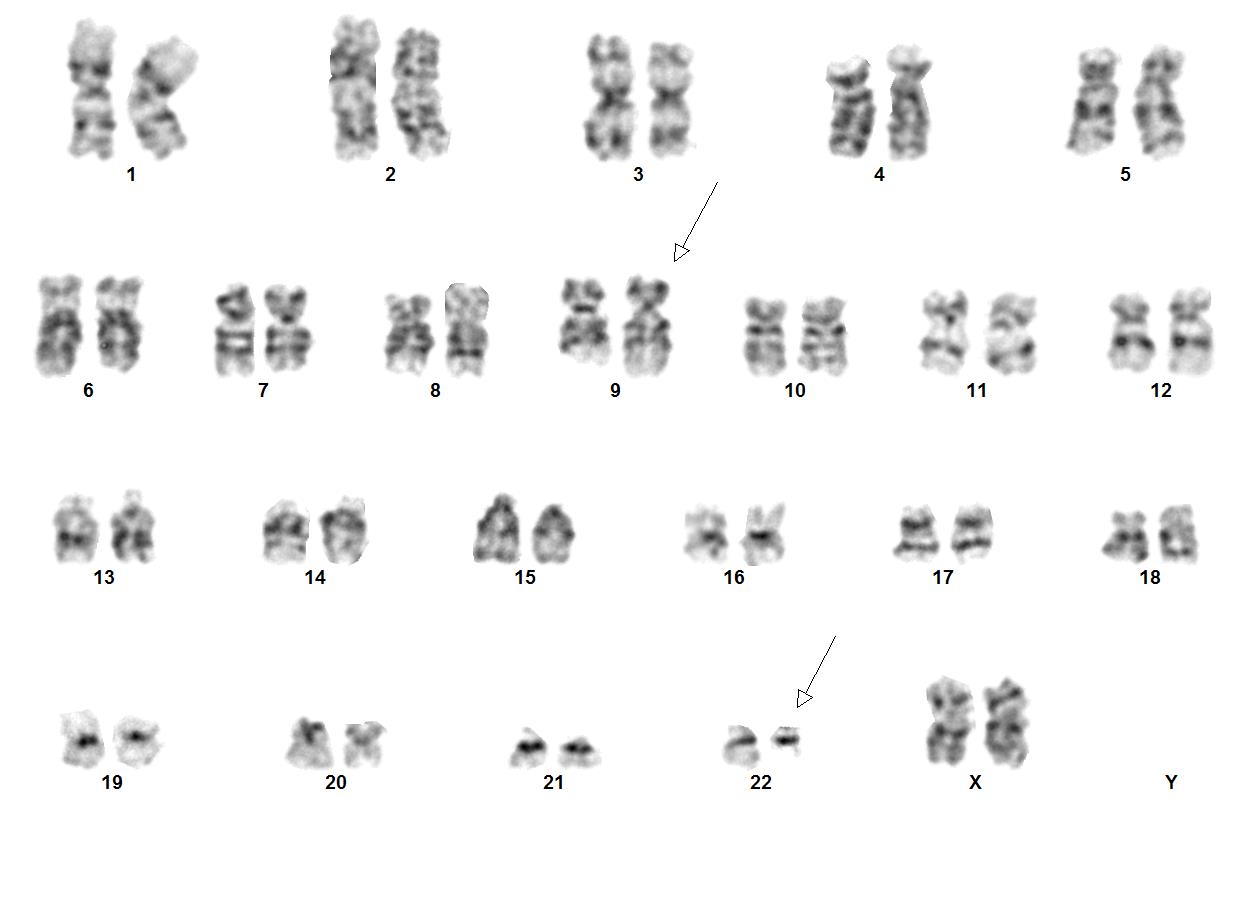
Chronic myeloid leukemia (CML) is a myeloproliferative neoplasm that is characterized by clonal expansion of predominantly granulocytic proliferation (neutrophils, eosinophils and basophils). The majority of the patients with CML are known to have a gene rearrangement called the Philadelphia Chromosome, which is a balanced genetic translocation t(9;22)(q34.1;q11.2) involving a fusion of the Abelson gene (ABL1) from chromosome 9q34 with the breakpoint cluster region (BCR) gene on chromosome 22q11.2. Althought 80% of the clonal evolution in CML cases can be attributed to classic Ph chromosome, secondary cytogenetic aberrations can be seen such as isochromosome 17q, gain of chromosome 8 or 19. ML was first recognized in 1845[1]. Nowell and Hungerford in 1960, who coined the term Philadelphia Chromosome after realizing consistent chromosomal abnormality in leukemic cells.[2] Later in 1973, the characteristic cytogenetic feature of CML was identified: reciprocal translocation of t(9;22)(q34.1;q11.2).[3] CML has the capacity to expand in both myeloid and lymphoid lineages. However, expansion is predominantly in the granulocyte compartment of the myeloid lineages in the bone marrow.[4] In the previous WHO 4th edition, CML was be divided into 3 phases of disease: chronic phase, accelerated phase and blastic phase. Currently, in the revised classification of CML, AP at diagnosis or during treatment has been omitted and replaced by recognising only the chronic and blast phases
Synonyms / Terminology
Formerly chronic myelogenous leukemia or chronic granulocytic leukemia
Epidemiology / Prevalence
Global annual incidence of CML is 1-2 cases per 100,000 population. CML has male predominance with male to female ratio of 1.4:1. The prevalence of CML is increasing due to successful Tyrosine kinase inhibitor (TKI) therapy. Predilection of CML among certain ethnic groups has not been reported.[5] Regional variations in age at diagnosis and overall survival among patients with chronic myeloid leukemia from low and middle income countries.[6] The median age of patients diagnosed with CML is 66 years according to Surveillance, Epidemiology, and End Results (SEER) program and Medical Research Council (MRC) data. Although the etiology of CML is largely unknown, cases of CML have been reported in association with radiation exposure. No studies have shown any genetic inheritance of CML.
Clinical Features
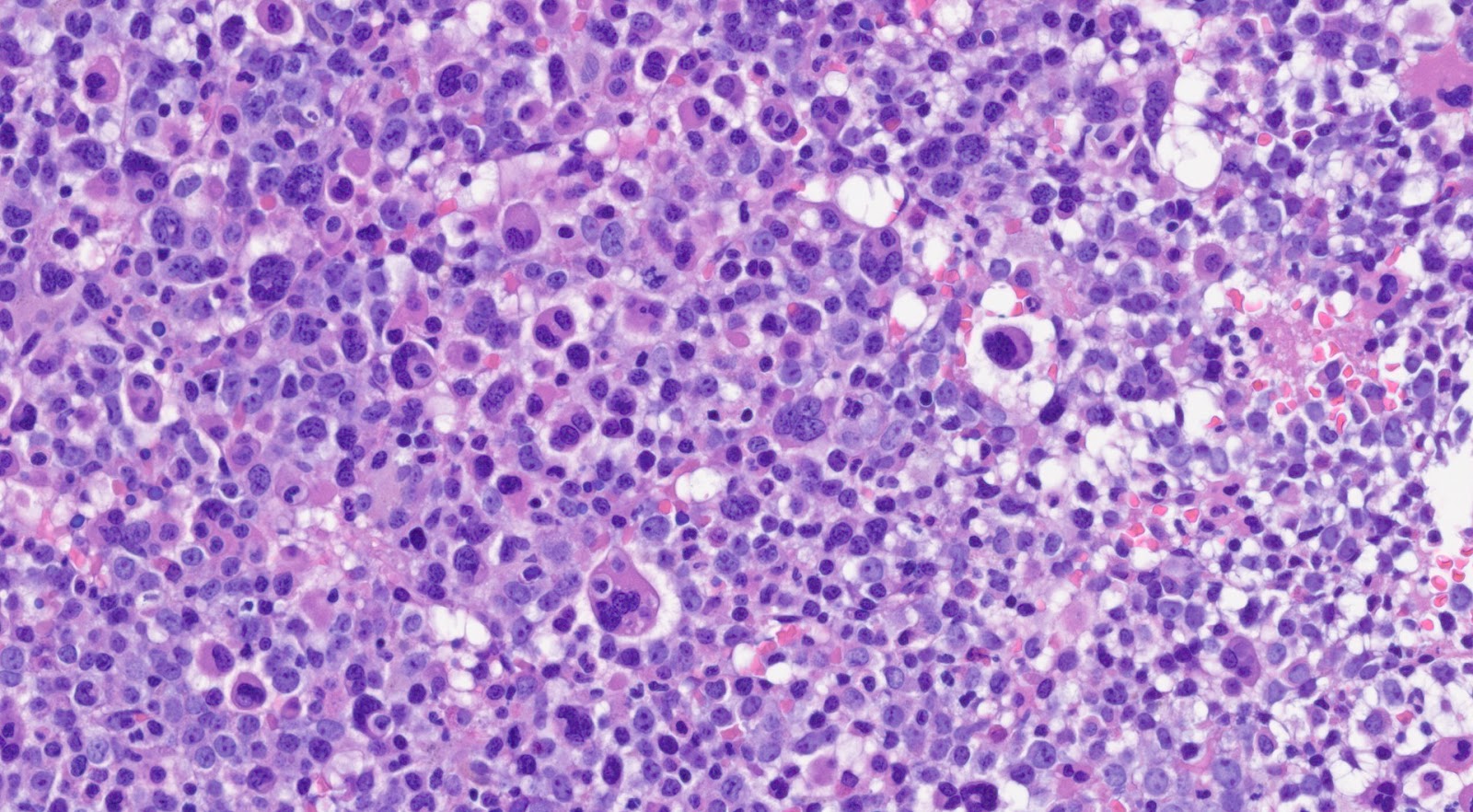
Approximately 50% of the patients who are diagnosed with CML are asymptomatic and diagnosed during the routine blood tests.[7] CML is a hematological disease that occurs predominantly in adults but in rare cases, it can occur in the pediatric population.[8] The onset of CML is insidious. Patients with CML usually experience dragging sensation of the abdomen due to splenomegaly. The clinical hallmark of CML is the uncontrolled proliferation of mature and maturing granulocytes at all stages of maturation: metamyelocytes, myelocytes, promyelocytes, and myeloblasts. Patients with CML usually begin with the initial chronic phase before entering the terminal blastic phase and 60-80% of the patients go through accelerated phase before reaching the terminal blastic phase. In chronic phase, CML patients show abnormal routine blood tests with clinical symptoms such as unintentional weight loss, loss of appetite, satiety, fatigue, insomnia and palpable splenomegaly. In rare cases, hyperviscosity syndrome can be a manifestation with a wide spectrum of features such as priapism, tinnitus, hearing loss, cerebral accidents and blindness. In the blastic phase, CML leukemic cells resemble acute leukemic cells morphologically. CNS and lymph node involvement are notable in the blastic phase of CML. If untreated, CML patients will progress to the terminal blastic phase in 3 to 5 years.
Sites of Involvement
Spleen is known to be the most common site of involvement as patients with CML usually present with splenomegaly. Literature has shown that bone marrow is always involved in patients with CML.
Morphologic Features
Morphologically, peripheral blood smear shows the classic features of chronic-phase CML: granulocytic leukocytosis with left shift, neutrophilia, no increase in blasts, myelocyte “bulge” and basophilia.[9] Granulocytes seen in CML patients usually lack dysplastic features. In bone marrow, the aspirate smear shows classic features of CML: marked increase in neutrophils and precursors with a myeloid: erythroid ratio of > 10:1 and small hypolobated megakaryocytes. CML can sometimes present with thrombocytosis in peripheral blood smear, mimicking essential thrombocythemia (ET). Most of the time, primary myelofibrosis (PMF) share overlapping morphological features with CML in the peripheral blood; one distinguishing feature is that the megakaryocyte morphology in PMF is large, bizarre, and hyperchromatic, which is the feature not seen in the CML.
Immunophenotype
The role of immunohistochemistry is minimal in diagnosing CML. CML usually shows blastic markers (CD 34, CD 117, TdT), which can be useful to confirm extramedullary (splenic involvement with blastic transformation). Lineage-specific markers (MPO, lysosome, CD42b, CD79a, PAX5, CD3) are helpful in distinguishing among myeloid, lymphoid or megakaryocytic transformation.
| Finding | Marker |
|---|---|
| Positive | CD34 |
| Positive | CD117 |
|
Positive |
Tdt |
Chromosomal Rearrangements (Gene Fusions)
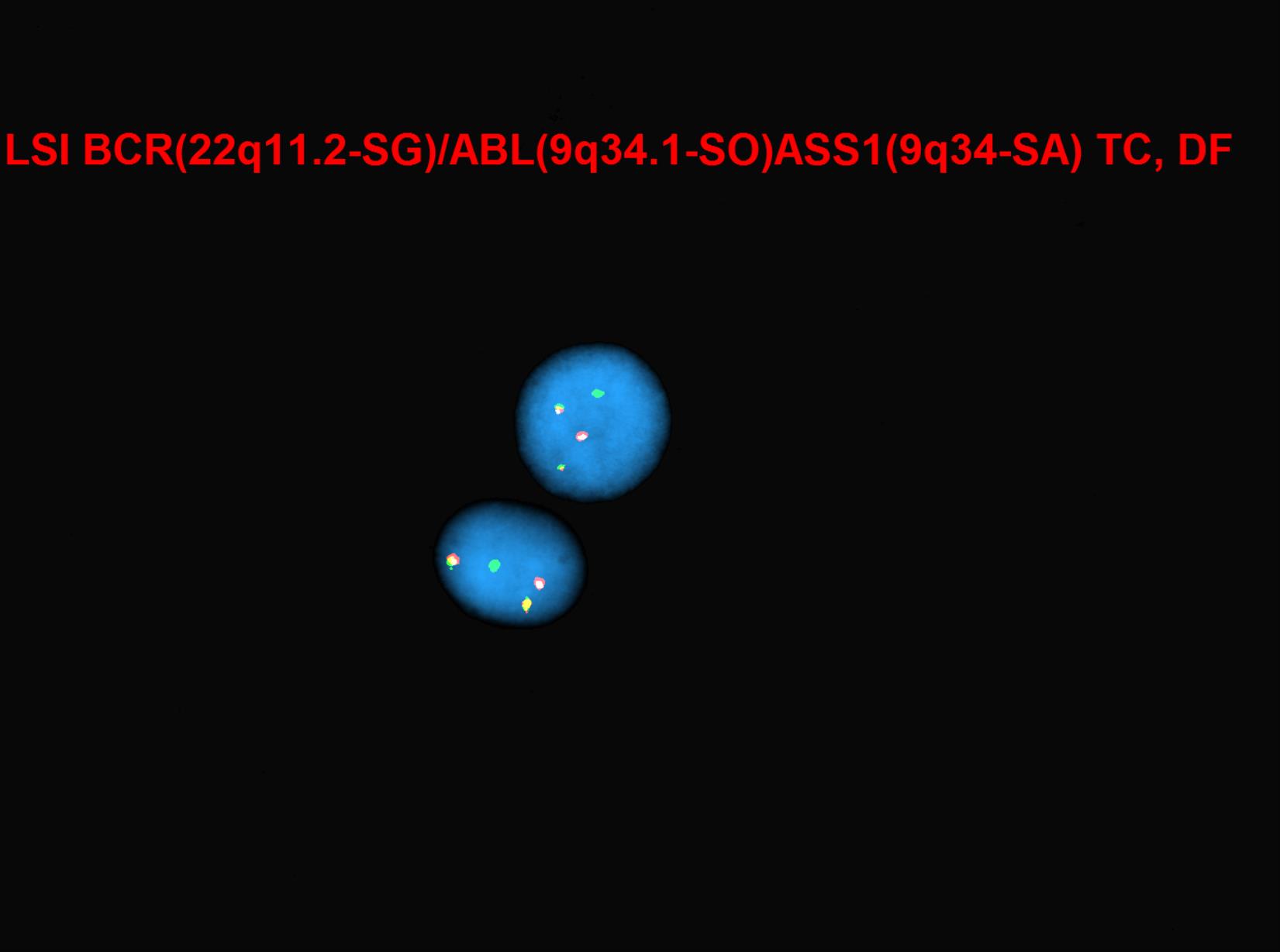
CML is the first cancer that is known to be linked to a specific genetic abnormality, namely the balanced chromosomal translocation known as Philadelphia Chromosome. A focal gene area of BCR (Breakpoint Cluster Region) from chromosome 22 is fused with another gene ABL (Tyrosine protein kinase ABL) that is located on chromosome 9. The chimeric oncogene BCR-ABL is the central to the pathology of CML because ABL carries a domain that is capable of phosphorylating tyrosine residues, activating a cascade of proteins that control the cell cycle. It was reported that 90% - 95% of the CML in chronic phase shows characteristic t(9;22)(q34;q11.2) reciprocal translocation that results in the Ph chromosome. This balanced translocation leads to the formation of the BCR/ABL fusion gene on chromosome 22 and a reciprocal ABL/BCR fusion gene on chromosome 9. Studies has shown that the latter gene ABL/BCR fusion gene does not seem to have any crucial role in CML and no ABL/BCR protein has been found.
| Chromosomal Rearrangement | Genes in Fusion (5’ or 3’ Segments) | Prevalence |
|---|---|---|
| t(9;22)(q34.1;q11.2) | 3'ABL1 / 5'BCR | More than 90% |
Characteristic Chromosomal Aberrations / Patterns
Atypical chronic myeloid leukemia (aCML) is a subtype of myelodysplastic/myeloproliferative neoplasm that lacks Philadelphia chromosome or rearrangements of PDGFRA, PDGFRB, or FGFR1. This hematological disorder has a considerable overlapping clinicopathological features with CML and CMML. It differs from CML by older median age, lower level of granulocytosis, multilineage dysplasia and lack of basophilia. Up until now, no cytogenetic changes have been associated with aCML. In peripheral blood smear, aCML typically shows granulocytic leukocytosis with striking neutrophil dysplasia (nuclear hyposegmentation and hypogranularity).
Genomic Gain/Loss/LOH
Not Applicable.
Gene Mutations (SNV/INDEL)
A few genes were noted to be altered during the transformed stages of CML, namely TP53, RB1, MYC, CDKN2A, NRAS, KRAS, RUNX1, MECOM, TET2, CBL, ASXL1, IDH1 and IDH2.
Epigenomics (Methylation)
Not Applicable.
Genes and Main Pathways Involved
Breakpoint Cluster Region protein (BCR) is encoded by BCR gene, located on chromosome 22. BCR protein has serine/threonine kinase activity.[7] The protein is also a GTPase-activating protein for p21rac and other kinases.[10] BCR protein is involved in the two main pathways: FGFR1 mutant receptor activation and G-protein signaling HRAS regulation pathway.[11] BCR-associated genetic rearrangement gives rise to hematological disorders. The ABL1 gene is located on chromosome 9q34.12 and encodes for ABL1 protein, which was discovered to be a tyrosine kinase protein.[12] Depending on the breakpoint of the BCR gene, the size of the fusion protein can vary: p190bcr-abl, p210bcr-abl, and p230bcr-abl, leading to three different isoforms.[7] BCR-ABL1 gene fusion encodes a chimeric protein, which is mostly 210 kDa(P210BCRABL1) with constitutive tyrosine-kinase activity, escaping the cytokine regulation and regulatory controls of many intracellular signaling pathways that are associated with proliferation, differentiation and apoptosis.[13][14] Many of the target proteins that are affected by dimerization of constitutive kinase activity of BCR-ABL fusion protein include STAT, RAS, RAF, JUN kinase, MYC, AKT, and other transducers.[15][16] It was shown that when CML progresses to the blastic crisis phase, a new additional mutation is acquired GSK3beta, which leads to the activation of beta-catenin, preventing myeloid cell lineages to mature.[17][18]
Diagnostic Testing Methods
Majority of the patients with CML are initially diagnosed through a blood test - complete blood count - before clinical manifestations. Bone marrow aspirate and core biopsy are performed sequentially to further support the process of making diagnosis as well as assessing percentage of blasts and basophils.[19] Histologic features of CML in the peripheral blood are helpful in deriving the CML diagnosis. Ancillary tests are performed to clinch the diagnosis: immunohistochemistry, flow cytometry, conventional cytogenetic analysis, FISH and molecular RT-PCR-based studies.
Cytogenetic testing is used in CML patients to monitor how patients are responding to the treatment by detecting the number of cells with the Philadelphia chromosome. In conjunction with cytogenetic testing, FISH and PCR are ordered to quantify treatment monitoring processes in CML. FISH allows the detection of BCR-ABL gene, which is essentially considered to be more of a sensitive test compared to cytogenetic testing. PCR is performed to find BCR-ABL fusion gene and other molecular abnormalities. PCR is very efficient because it can detect even one abnormal cell from approximately 1 million healthy cells. FISH can be used in rare cases where molecular transcripts are not detected.
Continuous monitoring is part of the standard of care in CML patients because it allows the clinicians to identify treatment failure, disease evolution and drug regimen adherence. Minimal residual disease monitoring is done by using RT-PCR. Major molecular response (MMR) is the critical goal of CML treatment. Complete or deep molecular response is achieved when there is absence of BCR-ABL1 transcript or >4.5 logs below baseline level.[20]
Clinical Significance (Diagnosis, Prognosis and Therapeutic Implications)
Diagnosis:
Currently four FDA approved tyrosine kinase inhibitors (TKIs) - imatinib, nilotinib, dasatinib and bosutinib - are the first line of treatment for patients with newly diagnosed CML in chronic phase (CML-CP).[21] For many years, inhibitors of the specific BCR-ABL1 tyrosine kinase are considered to be the most effective targeted therapy. A subset of CML patients can demonstrate resistance to TKI therapy through mutations in ABL1 and other mechanisms. The culprit of the resistance to TKI therapy can be attributed to so-called leukemic stem cells (LSCs), pluripotent BCR-ABL1+ progenitors that are largely quiescent.[22] Therefore, understanding of signaling pathways related to survival of LSCs may be helpful.
Prognosis: Acquired resistance to imatinib therapy , mostly with mutation in BCR-ABL kinase domain, is known to be associated with poor prognosis.[23] Five prognostic factors were shown to be associated with major cytogenetic response: the absence of blasts in peripheral blood, a hemoglobin level of more than 12 g per deciliter, the presence of less than 5 percent blasts in marrow, a time from diagnosis of CML to start of treatment of less than one year, and a history of cytogenetic relapse during interferon therapy.[24]
Therapeutic implication: Studies have shown that median survival
Complete cytogenetic response is defined as 0% of Philadelphia-chromosome (Ph)-positive cells in metaphase in bone marrow.[25]
Familial Forms
Not Applicable.
Links
References
- ↑ Jm, Goldman; et al. (2003). "Chronic Myeloid Leukemia--Advances in Biology and New Approaches to Treatment". PMID 14534339.
- ↑ Nowell, Peter C. (2007). "Discovery of the Philadelphia chromosome: a personal perspective". Journal of Clinical Investigation. 117 (8): 2033–2035. doi:10.1172/JCI31771. ISSN 0021-9738. PMC 1934591. PMID 17671636.
- ↑ Jd, Rowley (1973). "Letter: A New Consistent Chromosomal Abnormality in Chronic Myelogenous Leukaemia Identified by Quinacrine Fluorescence and Giemsa Staining". PMID 4126434.
- ↑ S, Faderl; et al. (1999). "The Biology of Chronic Myeloid Leukemia". PMID 10403855.
- ↑ Jv, Melo; et al. (1993). "The ABL-BCR Fusion Gene Is Expressed in Chronic Myeloid Leukemia". PMID 8417787.
- ↑ Am, Mendizabal; et al. (2013). "Regional Variations in Age at Diagnosis and Overall Survival Among Patients With Chronic Myeloid Leukemia From Low and Middle Income Countries". PMID 23411044.
- ↑ 7.0 7.1 7.2 Silver RT. Molecular Biology of CML. In: Kufe DW, Pollock RE, Weichselbaum RR, et al., editors. Holland-Frei Cancer Medicine. 6th edition. Hamilton (ON): BC Decker; 2003. Available from: https://www.ncbi.nlm.nih.gov/books/NBK13554/
- ↑ Am, Mendizabal; et al. (2013). "Regional Variations in Age at Diagnosis and Overall Survival Among Patients With Chronic Myeloid Leukemia From Low and Middle Income Countries". PMID 23411044.
- ↑ Vardiman JW, et al., (2016). Chronic myeloid leukaemia, BCR-ABL1-positive, in World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues, Revised 4th edition. Swerdlow SH, Campo E, Harris NL, et al., Editors. IARC Press: Lyon, France, p30-36.
- ↑ "BCR BCR activator of RhoGEF and GTPase [Homo sapiens (human)] - Gene - NCBI".
- ↑ Mn, Peiris; et al. (2019). "BCR: A Promiscuous Fusion Partner in Hematopoietic Disorders". doi:10.18632/oncotarget.26837. PMC 6505627. PMID 31105873.CS1 maint: PMC format (link)
- ↑ B, Chereda; et al. (2015). "Natural Course and Biology of CML". PMID 25814077.
- ↑ Jb, Konopka; et al. (1984). "An Alteration of the Human C-Abl Protein in K562 Leukemia Cells Unmasks Associated Tyrosine Kinase Activity". PMID 6204766.
- ↑ R, Ren (2005). "Mechanisms of BCR-ABL in the Pathogenesis of Chronic Myelogenous Leukaemia". PMID 15719031.
- ↑ S, Faderl; et al. (1999). "The Biology of Chronic Myeloid Leukemia". PMID 10403855.
- ↑ Cl, Sawyers (1999). "Chronic Myeloid Leukemia". PMID 10219069.
- ↑ Ch, Jamieson; et al. (2004). "Granulocyte-macrophage Progenitors as Candidate Leukemic Stem Cells in Blast-Crisis CML". PMID 15306667.
- ↑ Ae, Abrahamsson; et al. (2009). "Glycogen Synthase Kinase 3beta Missplicing Contributes to Leukemia Stem Cell Generation". doi:10.1073/pnas.0900189106. PMC 2646624. PMID 19237556.CS1 maint: PMC format (link)
- ↑ E, Jabbour; et al. (2018). "Chronic Myeloid Leukemia: 2018 Update on Diagnosis, Therapy and Monitoring". PMID 29411417.
- ↑ gknation (2015). "Treatment Outcomes".
- ↑ Ja, Kennedy; et al. (2018). "Tyrosine Kinase Inhibitors in the Treatment of Chronic-Phase CML: Strategies for Frontline Decision-making". doi:10.1007/s11899-018-0449-7. PMC 6023770. PMID 29687320.CS1 maint: PMC format (link)
- ↑ S, Tabarestani; et al. (2016). "New Developments in Chronic Myeloid Leukemia: Implications for Therapy". doi:10.17795/ijcp-3961. PMC 4922205. PMID 27366312.CS1 maint: PMC format (link)
- ↑ S, Branford; et al. (2003). "Detection of BCR-ABL Mutations in Patients With CML Treated With Imatinib Is Virtually Always Accompanied by Clinical Resistance, and Mutations in the ATP Phosphate-Binding Loop (P-loop) Are Associated With a Poor Prognosis". PMID 12623848.
- ↑ H, Kantarjian; et al. (2002). "Hematologic and Cytogenetic Responses to Imatinib Mesylate in Chronic Myelogenous Leukemia". PMID 11870241.
- ↑ J, Cortes; et al. (2011). "Monitoring Molecular Response in Chronic Myeloid Leukemia". doi:10.1002/cncr.25527. PMC 4969001. PMID 20960522.CS1 maint: PMC format (link)
Notes
*Primary authors will typically be those that initially create and complete the content of a page. If a subsequent user modifies the content and feels the effort put forth is of high enough significance to warrant listing in the authorship section, please contact the CCGA coordinators (contact information provided on the homepage). Additional global feedback or concerns are also welcome.