Difference between revisions of "HAEM5:Acute myeloid leukaemia with NUP98 rearrangement"
Templates/files updated (unreviewed pages in bold): Template:Cite journal, Module:Citation/CS1, Module:Citation/CS1/Configuration, Module:Citation/CS1/Whitelist, Module:Citation/CS1/Utilities, Module:Citation/CS1/Date validation, Module:Citation/CS1/Identifiers, Module:Citation/CS1/COinS, Module:Citation/CS1/styles.css, HAEM5:Acute myeloid leukaemia with NUP98 rearrangement, File:NUP98 NSD1.png, File:T(5;11).jpg
| [checked revision] | [pending revision] |
(Undo revision 15555 by Fatma.Al-Bulushi (talk)) Tag: Undo |
Bailey.Glen (talk | contribs) |
||
| (7 intermediate revisions by 2 users not shown) | |||
| Line 4: | Line 4: | ||
{{Under Construction}} | {{Under Construction}} | ||
| − | <span style="color:#0070C0">(General Instructions – The | + | <span style="color:#0070C0">(General Instructions – The focus of these pages is the clinically significant genetic alterations in each disease type. This is based on up-to-date knowledge from multiple resources such as PubMed and the WHO classification books. The CCGA is meant to be a supplemental resource to the WHO classification books; the CCGA captures in a continually updated wiki-stye manner the current genetics/genomics knowledge of each disease, which evolves more rapidly than books can be revised and published. If the same disease is described in multiple WHO classification books, the genetics-related information for that disease will be consolidated into a single main page that has this template (other pages would only contain a link to this main page). Use [https://www.genenames.org/ <u>HUGO-approved gene names and symbols</u>] (italicized when appropriate), [https://varnomen.hgvs.org/ <u>HGVS-based nomenclature for variants</u>], as well as generic names of drugs and testing platforms or assays if applicable. Please complete tables whenever possible and do not delete them (add N/A if not applicable in the table and delete the examples); to add (or move) a row or column in a table, click nearby within the table and select the > symbol that appears. Please do not delete or alter the section headings. The use of bullet points alongside short blocks of text rather than only large paragraphs is encouraged. Additional instructions below in italicized blue text should not be included in the final page content. Please also see </span><u>[[Author_Instructions]]</u><span style="color:#0070C0"> and [[Frequently Asked Questions (FAQs)|<u>FAQs</u>]] as well as contact your [[Leadership|<u>Associate Editor</u>]] or [mailto:CCGA@cancergenomics.org <u>Technical Support</u>].)</span> |
==Primary Author(s)*== | ==Primary Author(s)*== | ||
| − | + | Eric McGinnis, MD | |
| − | |||
| − | |||
| + | Fatma Albulushi, MD | ||
==WHO Classification of Disease== | ==WHO Classification of Disease== | ||
| Line 34: | Line 33: | ||
|} | |} | ||
| − | == | + | ==WHO Essential and Desirable Genetic Diagnostic Criteria.== |
| − | + | {| class="wikitable" | |
| − | + | |WHO Essential Criteria (Genetics)* | |
| − | + | |Detection of NUP98 rearrangement | |
| − | == | + | |- |
| − | + | |WHO Desirable Criteria (Genetics)* | |
| − | + | |Identification of the NUP98 fusion partner | |
| − | + | |- | |
| − | + | |Other Classification | |
| − | + | |Myeloid blast count may <20% | |
| − | + | |} | |
| − | + | <nowiki>*</nowiki>Note: These are only the genetic/genomic criteria. Additional diagnostic criteria can be found in the WHO Classification of Tumours. | |
| − | + | ==Related Terminology== | |
| − | + | <span style="color:#0070C0">(''Instructions: The table will have the related terminology from the WHO <u>autocompleted</u>.)''</span> | |
| − | |||
{| class="wikitable" | {| class="wikitable" | ||
| − | | | + | |+ |
| − | | | + | |Acceptable |
| − | + | | | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
|- | |- | ||
| − | | | + | |Not Recommended |
| − | | | + | | |
| − | |||
| − | |||
|} | |} | ||
| − | == | + | ==Gene Rearrangements== |
| + | Put your text here and fill in the table <span style="color:#0070C0">(''Instructions: Details on clinical significance such as prognosis and other important information can be provided in the notes section. Please include references throughout the table. Do not delete the table.'')</span> | ||
| + | {| class="wikitable sortable" | ||
| + | |- | ||
| + | !Driver Gene!!Fusion(s) and Common Partner Genes!!Molecular Pathogenesis!!Typical Chromosomal Alteration(s) | ||
| + | !Prevalence -Common >20%, Recurrent 5-20% or Rare <5% (Disease) | ||
| + | !Diagnostic, Prognostic, and Therapeutic Significance - D, P, T | ||
| + | !Established Clinical Significance Per Guidelines - Yes or No (Source) | ||
| + | !Clinical Relevance Details/Other Notes | ||
| + | |- | ||
| + | |<span class="blue-text">EXAMPLE:</span> ''ABL1''||<span class="blue-text">EXAMPLE:</span> ''BCR::ABL1''||<span class="blue-text">EXAMPLE:</span> The pathogenic derivative is the der(22) resulting in fusion of 5’ BCR and 3’ABL1.||<span class="blue-text">EXAMPLE:</span> t(9;22)(q34;q11.2) | ||
| + | |<span class="blue-text">EXAMPLE:</span> Common (CML) | ||
| + | |<span class="blue-text">EXAMPLE:</span> D, P, T | ||
| + | |<span class="blue-text">EXAMPLE:</span> Yes (WHO, NCCN) | ||
| + | |<span class="blue-text">EXAMPLE:</span> | ||
| + | The t(9;22) is diagnostic of CML in the appropriate morphology and clinical context (add reference). This fusion is responsive to targeted therapy such as Imatinib (Gleevec) (add reference). BCR::ABL1 is generally favorable in CML (add reference). | ||
| + | |- | ||
| + | |<span class="blue-text">EXAMPLE:</span> ''CIC'' | ||
| + | |<span class="blue-text">EXAMPLE:</span> ''CIC::DUX4'' | ||
| + | |<span class="blue-text">EXAMPLE:</span> Typically, the last exon of ''CIC'' is fused to ''DUX4''. The fusion breakpoint in ''CIC'' is usually intra-exonic and removes an inhibitory sequence, upregulating ''PEA3'' genes downstream of ''CIC'' including ''ETV1'', ''ETV4'', and ''ETV5''. | ||
| + | |<span class="blue-text">EXAMPLE:</span> t(4;19)(q25;q13) | ||
| + | |<span class="blue-text">EXAMPLE:</span> Common (CIC-rearranged sarcoma) | ||
| + | |<span class="blue-text">EXAMPLE:</span> D | ||
| + | | | ||
| + | |<span class="blue-text">EXAMPLE:</span> | ||
| − | + | ''DUX4'' has many homologous genes; an alternate translocation in a minority of cases is t(10;19), but this is usually indistinguishable from t(4;19) by short-read sequencing (add references). | |
| + | |- | ||
| + | |<span class="blue-text">EXAMPLE:</span> ''ALK'' | ||
| + | |<span class="blue-text">EXAMPLE:</span> ''ELM4::ALK'' | ||
| − | |||
| − | + | Other fusion partners include ''KIF5B, NPM1, STRN, TFG, TPM3, CLTC, KLC1'' | |
| + | |<span class="blue-text">EXAMPLE:</span> Fusions result in constitutive activation of the ''ALK'' tyrosine kinase. The most common ''ALK'' fusion is ''EML4::ALK'', with breakpoints in intron 19 of ''ALK''. At the transcript level, a variable (5’) partner gene is fused to 3’ ''ALK'' at exon 20. Rarely, ''ALK'' fusions contain exon 19 due to breakpoints in intron 18. | ||
| + | |<span class="blue-text">EXAMPLE:</span> N/A | ||
| + | |<span class="blue-text">EXAMPLE:</span> Rare (Lung adenocarcinoma) | ||
| + | |<span class="blue-text">EXAMPLE:</span> T | ||
| + | | | ||
| + | |<span class="blue-text">EXAMPLE:</span> | ||
| − | + | Both balanced and unbalanced forms are observed by FISH (add references). | |
| − | |||
| − | |||
| − | |||
| − | |||
|- | |- | ||
| − | + | |<span class="blue-text">EXAMPLE:</span> ''ABL1'' | |
| + | |<span class="blue-text">EXAMPLE:</span> N/A | ||
| + | |<span class="blue-text">EXAMPLE:</span> Intragenic deletion of exons 2–7 in ''EGFR'' removes the ligand-binding domain, resulting in a constitutively active tyrosine kinase with downstream activation of multiple oncogenic pathways. | ||
| + | |<span class="blue-text">EXAMPLE:</span> N/A | ||
| + | |<span class="blue-text">EXAMPLE:</span> Recurrent (IDH-wildtype Glioblastoma) | ||
| + | |<span class="blue-text">EXAMPLE:</span> D, P, T | ||
| + | | | ||
| + | | | ||
|- | |- | ||
| − | | | + | | |
| − | | | + | | |
| − | | | + | | |
| − | | | + | | |
| − | | | + | | |
| − | | | + | | |
| − | | | + | | |
| + | | | ||
|} | |} | ||
| − | == | + | Acute myeloid leukaemia (AML) with NUP98 rearrangement is characterized by chromosomal translocations involving NUP98 (nucleoporin 98 kDa) on chromosome 11p15.4 and various partner genes. (Reference WHO book). There are over 40 fusion partners which have been reported to date. NUP98 fusions can be categorized into three broad parts. The first category includes NUP98 fusions with transcription factors as partners, which can change the expression of target genes through DNA binding domains. The second category is NUP98 fusions with epigenetic modifiers that modify chromatin to change target gene expression. The third category of NUP98 fusions has neither the DNA binding nor chromatin remodeling domain.<ref name=":0">{{Cite journal|last=Mohanty|first=Sagarajit|date=2023-09|title=NUP98 Rearrangements in AML: Molecular Mechanisms and Clinical Implications|url=https://www.mdpi.com/2673-7523/3/3/11|journal=Onco|language=en|volume=3|issue=3|pages=147–164|doi=10.3390/onco3030011|issn=2673-7523}}</ref> |
| − | |||
| − | {| class="wikitable | + | The NUP98 gene (chromosome 11p15) encodes a nucleoporin protein, which is part of the nuclear pore complex which regulates nucleocytoplasmic transport of protein and RNA. NUP98 fusion proteins involve the N-terminal portion of NUP98 and the C-terminal portion of the fusion partner. These fusion partners consist of homeodomain proteins, which are transcription factors, and non-homeodomain proteins, which are thought to play a role in transcriptional or epigenetic regulation.<ref name=":0" /><ref name=":1">{{Cite journal|last=Bertrums|first=Eline J. M.|last2=Smith|first2=Jenny L.|last3=Harmon|first3=Lauren|last4=Ries|first4=Rhonda E.|last5=Wang|first5=Yi-Cheng J.|last6=Alonzo|first6=Todd A.|last7=Menssen|first7=Andrew J.|last8=Chisholm|first8=Karen M.|last9=Leonti|first9=Amanda R.|date=2023-02-23|title=Comprehensive molecular and clinical characterization of NUP98 fusions in pediatric acute myeloid leukemia|url=https://www.haematologica.org/article/view/haematol.2022.281653|journal=Haematologica|language=en|volume=108|issue=8|pages=2044–2058|doi=10.3324/haematol.2022.281653|issn=1592-8721}}</ref> |
| + | <br /> | ||
| + | {| class="wikitable" | ||
| + | |'''Driver Gene''' | ||
| + | |'''Fusion(s) and Common Partner Genes''' | ||
| + | |'''Molecular Pathogenesis''' | ||
| + | |'''Typical Chromosomal Alteration(s)''' | ||
| + | |'''Prevalence -Common >20%, Recurrent 5-20% or Rare <5% (Disease)''' | ||
| + | |'''Diagnostic, Prognostic, and Therapeutic Significance - D, P, T''' | ||
| + | |'''Established Clinical Significance Per Guidelines - Yes or No (Source)''' | ||
| + | |'''Clinical Relevance Details/Other Notes''' | ||
|- | |- | ||
| − | + | |''NUP98'' | |
| − | + | |''NUP98::NSD1'' | |
| − | + | <br /> | |
| − | + | |NUP98-NSD1 prevents EZH2-mediated repression of Hox-A locus genes by colocalizing H3K36 methylation and histone acetylation at regulatory DNA elements hence preventing myeloid progenitor immortalization. | |
| − | + | |t(5;11)(q35;p15) | |
| + | |||
| + | Usually cryptic | ||
| + | |Rare (AML) | ||
| + | |Defining genetic abnormality in AML | ||
| + | |Yes (WHO) | ||
| + | |Rare but recurrent alteration seen mainly in children and young adults with AML. Poor overall survival, disease free survival, induction failure and chemotherapy resistance.<ref name=":1" /> | ||
|- | |- | ||
| − | | | + | |''NUP98'' |
| − | + | |''NUP98::KDM5A'' | |
| − | + | |KDM5A is an epigenetic-modifying partners of NUP98 which dysregulate Hox genes expression through recognition of H3K4me3/2 marks by the plant homeodomain (PHD) finger domain. | |
| − | + | |t(11;12)(p15;p13) | |
| − | |||
| − | |||
| − | + | Usually cryptic | |
| − | + | |Rare (AML) | |
| − | + | |Defining genetic abnormality in AML | |
| − | = | + | |Yes (WHO) |
| + | |''Commonly associated with erythroid and megakaryocytic phenotypes in pediatric AML (acute erythroid leukemia and acute megakaryocytic leukemia).''<ref name=":1" /> | ||
| − | + | ''Usually associate with unfavorable outcomes'' | |
| + | |- | ||
| + | |''NUP98'' | ||
| + | |''NUP98::HOXA9'' | ||
| + | <br /> | ||
| + | |NUP98 fusions bind near the HOX genes loci and activate their expression through chromatin remodeling. The overexpression of distal HoxA cluster genes promote self-renewal and drive leukogenesis. | ||
| + | |t(7;11)(p15, p15) | ||
| + | |Rare (AML) | ||
| + | |Defining genetic abnormality in AML | ||
| + | | | ||
| + | | | ||
| + | |} | ||
| + | ==Individual Region Genomic Gain/Loss/LOH== | ||
| + | Put your text here and fill in the table <span style="color:#0070C0">(''Instructions: Includes aberrations not involving gene rearrangements. Details on clinical significance such as prognosis and other important information can be provided in the notes section. Can refer to CGC workgroup tables as linked on the homepage if applicable. Please include references throughout the table. Do not delete the table.'') </span> | ||
{| class="wikitable sortable" | {| class="wikitable sortable" | ||
|- | |- | ||
| − | !Chr #!!Gain | + | !Chr #!!'''Gain, Loss, Amp, LOH'''!!'''Minimal Region Cytoband and/or Genomic Coordinates [Genome Build; Size]'''!!'''Relevant Gene(s)''' |
| − | !Diagnostic | + | !'''Diagnostic, Prognostic, and Therapeutic Significance - D, P, T''' |
| − | + | !'''Established Clinical Significance Per Guidelines - Yes or No (Source)''' | |
| − | ! | + | !'''Clinical Relevance Details/Other Notes''' |
| − | !Notes | ||
|- | |- | ||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> | ||
| − | |||
7 | 7 | ||
|<span class="blue-text">EXAMPLE:</span> Loss | |<span class="blue-text">EXAMPLE:</span> Loss | ||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> | ||
| − | + | chr7 | |
| − | chr7 | ||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> | ||
| − | + | Unknown | |
| − | + | |<span class="blue-text">EXAMPLE:</span> D, P | |
| − | + | |<span class="blue-text">EXAMPLE:</span> No | |
| − | | | ||
| − | |No | ||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> | ||
| − | + | Presence of monosomy 7 (or 7q deletion) is sufficient for a diagnosis of AML with MDS-related changes when there is ≥20% blasts and no prior therapy (add reference). Monosomy 7/7q deletion is associated with a poor prognosis in AML (add references). | |
| − | Presence of monosomy 7 (or 7q deletion) is sufficient for a diagnosis of AML with MDS-related changes when there is ≥20% blasts and no prior therapy (add reference). Monosomy 7/7q deletion is associated with a poor prognosis in AML (add | ||
|- | |- | ||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> | ||
| − | |||
8 | 8 | ||
|<span class="blue-text">EXAMPLE:</span> Gain | |<span class="blue-text">EXAMPLE:</span> Gain | ||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> | ||
| + | chr8 | ||
| + | |<span class="blue-text">EXAMPLE:</span> | ||
| + | Unknown | ||
| + | |<span class="blue-text">EXAMPLE:</span> D, P | ||
| + | | | ||
| + | |<span class="blue-text">EXAMPLE:</span> | ||
| + | Common recurrent secondary finding for t(8;21) (add references). | ||
| + | |- | ||
| + | |<span class="blue-text">EXAMPLE:</span> | ||
| + | 17 | ||
| + | |<span class="blue-text">EXAMPLE:</span> Amp | ||
| + | |<span class="blue-text">EXAMPLE:</span> | ||
| + | 17q12; chr17:39,700,064-39,728,658 [hg38; 28.6 kb] | ||
| + | |<span class="blue-text">EXAMPLE:</span> | ||
| + | ''ERBB2'' | ||
| + | |<span class="blue-text">EXAMPLE:</span> D, P, T | ||
| + | | | ||
| + | |<span class="blue-text">EXAMPLE:</span> | ||
| + | Amplification of ''ERBB2'' is associated with HER2 overexpression in HER2 positive breast cancer (add references). Add criteria for how amplification is defined. | ||
| + | |- | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |} | ||
| − | + | No characteristic chromosomal gain or loss. However, trisomy 8 and chromosome 13 abnormalities may be observed. | |
| − | |||
| − | + | Several reports indicated that del(13q) is a frequent event in ''NUP98::KDM5A'' AML patients, indicating co-occurrence of ''NUP98-KDMA'' fusion with ''RB1'' deletion. | |
| + | <br /> | ||
| + | {| class="wikitable" | ||
| + | |'''Chromosome Number''' | ||
| + | |'''Gain/Loss/Amp/LOH''' | ||
| + | |'''Minimal Region Cytoband and/or Genomic Coordinates [Genome Build; Size]''' | ||
| + | |'''Relevant Gene(s)''' | ||
| + | |'''Diagnostic, Prognostic, and Therapeutic Significance - D, P, T''' | ||
| + | |'''Established Clinical Significance Per Guidelines - Yes or No (Source)''' | ||
| + | |'''Clinical Relevance Details/Other Notes''' | ||
| + | |- | ||
| + | |8 | ||
| + | |Gain | ||
| + | |Trisomy 8 | ||
| + | |Unknown | ||
| + | |NA | ||
|No | |No | ||
| − | | | + | | |
| − | | | + | |- |
| − | | | + | |13 |
| + | |loss | ||
| + | |Deletion of 13q | ||
| + | |RB1 gene | ||
| + | |NA | ||
| + | | | ||
| + | |Particularly associated with NUP98::KDM5A | ||
| + | |} | ||
| − | + | ==Characteristic Chromosomal or Other Global Mutational Patterns== | |
| − | |||
| − | ==Characteristic Chromosomal Patterns== | ||
| − | |||
| + | Put your text here and fill in the table <span style="color:#0070C0">(I''nstructions: Included in this category are alterations such as hyperdiploid; gain of odd number chromosomes including typically chromosome 1, 3, 5, 7, 11, and 17; co-deletion of 1p and 19q; complex karyotypes without characteristic genetic findings; chromothripsis; microsatellite instability; homologous recombination deficiency; mutational signature pattern; etc. Details on clinical significance such as prognosis and other important information can be provided in the notes section. Please include references throughout the table. Do not delete the table.'')</span> | ||
{| class="wikitable sortable" | {| class="wikitable sortable" | ||
|- | |- | ||
!Chromosomal Pattern | !Chromosomal Pattern | ||
| − | ! | + | !Molecular Pathogenesis |
| − | !Prognostic Significance | + | !'''Prevalence -''' |
| − | ! | + | '''Common >20%, Recurrent 5-20% or Rare <5% (Disease)''' |
| − | !Notes | + | !'''Diagnostic, Prognostic, and Therapeutic Significance - D, P, T''' |
| + | !'''Established Clinical Significance Per Guidelines - Yes or No (Source)''' | ||
| + | !'''Clinical Relevance Details/Other Notes''' | ||
|- | |- | ||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> | ||
| − | |||
Co-deletion of 1p and 18q | Co-deletion of 1p and 18q | ||
| − | | | + | |<span class="blue-text">EXAMPLE:</span> See chromosomal rearrangements table as this pattern is due to an unbalanced derivative translocation associated with oligodendroglioma (add reference). |
| − | | | + | |<span class="blue-text">EXAMPLE:</span> Common (Oligodendroglioma) |
| − | | | + | |<span class="blue-text">EXAMPLE:</span> D, P |
| + | | | ||
| + | | | ||
| + | |- | ||
|<span class="blue-text">EXAMPLE:</span> | |<span class="blue-text">EXAMPLE:</span> | ||
| − | + | Microsatellite instability - hypermutated | |
| − | + | | | |
| + | |<span class="blue-text">EXAMPLE:</span> Common (Endometrial carcinoma) | ||
| + | |<span class="blue-text">EXAMPLE:</span> P, T | ||
| + | | | ||
| + | | | ||
| + | |- | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
|} | |} | ||
| − | ==Gene Mutations (SNV / INDEL)== | + | ==Gene Mutations (SNV/INDEL)== |
| − | + | Put your text here and fill in the table <span style="color:#0070C0">(''Instructions: This table is not meant to be an exhaustive list; please include only genes/alterations that are recurrent or common as well either disease defining and/or clinically significant. If a gene has multiple mechanisms depending on the type or site of the alteration, add multiple entries in the table. For clinical significance, denote associations with FDA-approved therapy (not an extensive list of applicable drugs) and NCCN or other national guidelines if applicable; Can also refer to CGC workgroup tables as linked on the homepage if applicable as well as any high impact papers or reviews of gene mutations in this entity. Details on clinical significance such as prognosis and other important information such as concomitant and mutually exclusive mutations can be provided in the notes section. Please include references throughout the table. Do not delete the table.'') </span> | |
| − | Put your text here and fill in the table <span style="color:#0070C0">(''Instructions: This table is not meant to be an exhaustive list; please include only genes/alterations that are recurrent | ||
| − | |||
{| class="wikitable sortable" | {| class="wikitable sortable" | ||
|- | |- | ||
| − | !Gene | + | !Gene!!'''Genetic Alteration'''!!'''Tumor Suppressor Gene, Oncogene, Other'''!!'''Prevalence -''' |
| − | !''' | + | '''Common >20%, Recurrent 5-20% or Rare <5% (Disease)''' |
| − | ! | + | !'''Diagnostic, Prognostic, and Therapeutic Significance - D, P, T ''' |
| − | + | !'''Established Clinical Significance Per Guidelines - Yes or No (Source)''' | |
| − | + | !'''Clinical Relevance Details/Other Notes''' | |
|- | |- | ||
| − | |<span class="blue-text">EXAMPLE:</span> | + | |<span class="blue-text">EXAMPLE:</span>''EGFR'' |
| − | <span class="blue-text">EXAMPLE:</span> | + | <br /> |
| + | |<span class="blue-text">EXAMPLE:</span> Exon 18-21 activating mutations | ||
| + | |<span class="blue-text">EXAMPLE:</span> Oncogene | ||
| + | |<span class="blue-text">EXAMPLE:</span> Common (lung cancer) | ||
| + | |<span class="blue-text">EXAMPLE:</span> T | ||
| + | |<span class="blue-text">EXAMPLE:</span> Yes (NCCN) | ||
| + | |<span class="blue-text">EXAMPLE:</span> Exons 18, 19, and 21 mutations are targetable for therapy. Exon 20 T790M variants cause resistance to first generation TKI therapy and are targetable by second and third generation TKIs (add references). | ||
| + | |- | ||
| + | |<span class="blue-text">EXAMPLE:</span> ''TP53''; Variable LOF mutations | ||
| + | <br /> | ||
| + | |<span class="blue-text">EXAMPLE:</span> Variable LOF mutations | ||
| + | |<span class="blue-text">EXAMPLE:</span> Tumor Supressor Gene | ||
| + | |<span class="blue-text">EXAMPLE:</span> Common (breast cancer) | ||
| + | |<span class="blue-text">EXAMPLE:</span> P | ||
| + | | | ||
| + | |<span class="blue-text">EXAMPLE:</span> >90% are somatic; rare germline alterations associated with Li-Fraumeni syndrome (add reference). Denotes a poor prognosis in breast cancer. | ||
| + | |- | ||
| + | |<span class="blue-text">EXAMPLE:</span> ''BRAF''; Activating mutations | ||
| + | |<span class="blue-text">EXAMPLE:</span> Activating mutations | ||
| + | |<span class="blue-text">EXAMPLE:</span> Oncogene | ||
| + | |<span class="blue-text">EXAMPLE:</span> Common (melanoma) | ||
| + | |<span class="blue-text">EXAMPLE:</span> T | ||
| + | | | ||
| + | | | ||
| + | |- | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | | | ||
| + | |}Note: A more extensive list of mutations can be found in [https://www.cbioportal.org/ <u>cBioportal</u>], [https://cancer.sanger.ac.uk/cosmic <u>COSMIC</u>], and/or other databases. When applicable, gene-specific pages within the CCGA site directly link to pertinent external content. | ||
| − | + | FLT3-ITD and WT1 mutation are recurring events in NUP98::NSD1 and was also observed in some NUP98::HOXA9 AML patients.(R1). Loss of RB1 at 13q14 is particularly associated with NUP98::KDM5A | |
| − | + | {| class="wikitable" | |
| − | + | |'''Gene''' | |
| − | | | + | |'''Genetic Alteration''' |
| − | | | + | |'''Tumor Suppressor Gene (TSG)/Oncogene/Other''' |
| − | + | |'''Prevalence -Common >20%, Recurrent 5-20% or Rare <5% (Disease)''' | |
| − | + | |'''Diagnostic, Prognostic, and Therapeutic Significance - D, P, T''' | |
| − | | | + | |'''Established Clinical Significance Per Guidelines - Yes or No (Source)''' |
| − | | | + | |'''Clinical Relevance Details/Other Notes''' |
| + | |- | ||
| + | |''FLT3-ITD'' | ||
| + | | | ||
| + | | | ||
| + | |Recurrent | ||
| + | |Poor prognosis | ||
| + | | | ||
| + | |Seen in 67 to 91% of cases with NUP98::NSD1 | ||
| + | |- | ||
| + | |''WT1'' | ||
| + | | | ||
| + | | | ||
| + | |Rare | ||
| + | | | ||
| + | | | ||
| + | |Reported in 33-55% of NUP98::NSD1 rearranged AML | ||
| + | |- | ||
| + | |''RB1'' | ||
| + | | | ||
| | | | ||
| + | |Rare | ||
| | | | ||
| | | | ||
| − | | | + | |Particularly associated with NUP98::KDM5A |
| − | |||
|} | |} | ||
| − | |||
==Epigenomic Alterations== | ==Epigenomic Alterations== | ||
| + | |||
Put your text here | Put your text here | ||
| + | ==Genes and Main Pathways Involved== | ||
| − | |||
| − | Put your text here and fill in the table <span style="color:#0070C0">(''Instructions: | + | Put your text here and fill in the table <span style="color:#0070C0">(''Instructions: Please include references throughout the table. Do not delete the table.)''</span> |
{| class="wikitable sortable" | {| class="wikitable sortable" | ||
|- | |- | ||
!Gene; Genetic Alteration!!Pathway!!Pathophysiologic Outcome | !Gene; Genetic Alteration!!Pathway!!Pathophysiologic Outcome | ||
|- | |- | ||
| − | |<span class="blue-text">EXAMPLE:</span> BRAF and MAP2K1; Activating mutations | + | |<span class="blue-text">EXAMPLE:</span> ''BRAF'' and ''MAP2K1''; Activating mutations |
|<span class="blue-text">EXAMPLE:</span> MAPK signaling | |<span class="blue-text">EXAMPLE:</span> MAPK signaling | ||
|<span class="blue-text">EXAMPLE:</span> Increased cell growth and proliferation | |<span class="blue-text">EXAMPLE:</span> Increased cell growth and proliferation | ||
|- | |- | ||
| − | |<span class="blue-text">EXAMPLE:</span> CDKN2A; Inactivating mutations | + | |<span class="blue-text">EXAMPLE:</span> ''CDKN2A''; Inactivating mutations |
|<span class="blue-text">EXAMPLE:</span> Cell cycle regulation | |<span class="blue-text">EXAMPLE:</span> Cell cycle regulation | ||
|<span class="blue-text">EXAMPLE:</span> Unregulated cell division | |<span class="blue-text">EXAMPLE:</span> Unregulated cell division | ||
|- | |- | ||
| − | |<span class="blue-text">EXAMPLE:</span> | + | |<span class="blue-text">EXAMPLE:</span> ''KMT2C'' and ''ARID1A''; Inactivating mutations |
| − | |<span class="blue-text">EXAMPLE:</span> | + | |<span class="blue-text">EXAMPLE:</span> Histone modification, chromatin remodeling |
| − | |<span class="blue-text">EXAMPLE:</span> | + | |<span class="blue-text">EXAMPLE:</span> Abnormal gene expression program |
| + | |- | ||
| + | | | ||
| + | | | ||
| + | | | ||
|} | |} | ||
==Genetic Diagnostic Testing Methods== | ==Genetic Diagnostic Testing Methods== | ||
| − | + | Rearrangements involving NUP98 are often cryptic on conventional karyotype, owing to terminal location of NUP98 on chromosome 11p15.4. Most patients have a normal karyotype. Diagnosis is established using the following tests: | |
| + | |||
| + | *FISH using NUP98 break-apart probes | ||
| + | *RT-PCR for fusion proteins like NUP98::NSD1 | ||
| + | *RNA sequencing | ||
| + | *Optical Genome Mapping (OGM) | ||
| + | |||
| + | <br /> | ||
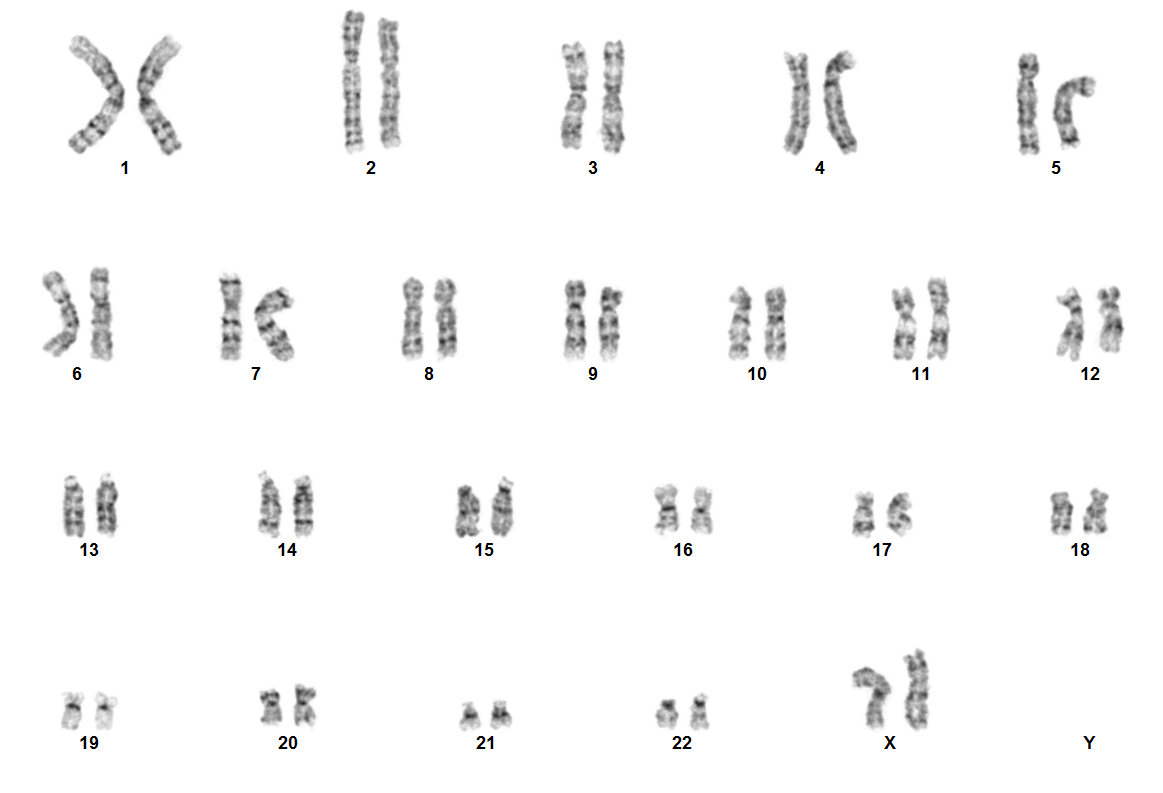
| + | [[File:NUP98 NSD1.png|none|thumb|617x617px|Karyotype image of NUP98 rearranged acute myeloid leukemia. Due to the cryptic nature of NUP98 rearrangement, karyotype is usually normal. ]] | ||
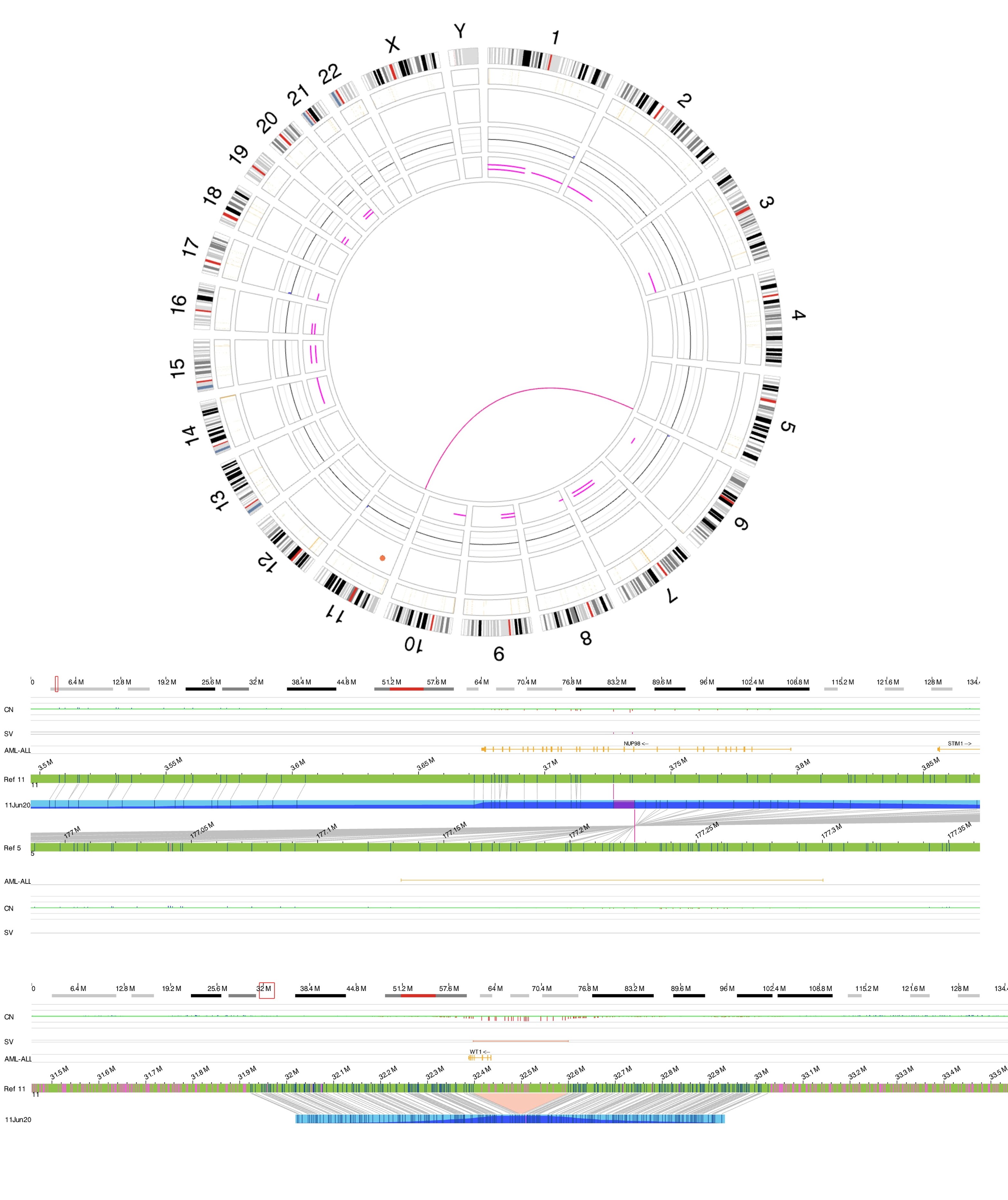
| + | [[File:T(5;11).jpg|none|thumb|584x584px|Optical genome mapping. Figure A showing circus plot with t(5;11). Figure B showing exact breakpoints of the translocation leading to NUP98::NSD1 fusion. Figure C showing WT1 deletion which is a common secondary event in NUP98 rearranged AML.]] | ||
| + | <br /> | ||
==Familial Forms== | ==Familial Forms== | ||
| + | |||
Put your text here <span style="color:#0070C0">(''Instructions: Include associated hereditary conditions/syndromes that cause this entity or are caused by this entity.'') </span> | Put your text here <span style="color:#0070C0">(''Instructions: Include associated hereditary conditions/syndromes that cause this entity or are caused by this entity.'') </span> | ||
| − | |||
==Additional Information== | ==Additional Information== | ||
| − | + | <br /> | |
==Links== | ==Links== | ||
| − | |||
| + | Put a link here or anywhere appropriate in this page <span style="color:#0070C0">(''Instructions: Highlight the text to which you want to add a link in this section or elsewhere, select the "Link" icon at the top of the wiki page, and search the name of the internal page to which you want to link this text, or enter an external internet address by including the "<nowiki>http://www</nowiki>." portion.'')</span> | ||
==References== | ==References== | ||
| − | |||
| − | |||
| − | # | + | (use the "Cite" icon at the top of the page) <span style="color:#0070C0">(''Instructions: Add each reference into the text above by clicking where you want to insert the reference, selecting the “Cite” icon at the top of the wiki page, and using the “Automatic” tab option to search by PMID to select the reference to insert. If a PMID is not available, such as for a book, please use the “Cite” icon, select “Manual” and then “Basic Form”, and include the entire reference. To insert the same reference again later in the page, select the “Cite” icon and “Re-use” to find the reference; DO NOT insert the same reference twice using the “Automatic” tab as it will be treated as two separate references. The reference list in this section will be automatically generated and sorted''</span><span style="color:#0070C0">''.''</span><span style="color:#0070C0">)</span> |
| + | ==Notes== | ||
| + | <nowiki>*</nowiki>Primary authors will typically be those that initially create and complete the content of a page. If a subsequent user modifies the content and feels the effort put forth is of high enough significance to warrant listing in the authorship section, please contact the [[Leadership|''<u>Associate Editor</u>'']] or other CCGA representative. When pages have a major update, the new author will be acknowledged at the beginning of the page, and those who contributed previously will be acknowledged below as a prior author. | ||
| − | + | Prior Author(s): | |
| − | + | ||
| + | |||
<nowiki>*</nowiki>''Citation of this Page'': “Acute myeloid leukaemia with NUP98 rearrangement”. Compendium of Cancer Genome Aberrations (CCGA), Cancer Genomics Consortium (CGC), updated {{REVISIONMONTH}}/{{REVISIONDAY}}/{{REVISIONYEAR}}, <nowiki>https://ccga.io/index.php/HAEM5:Acute_myeloid_leukaemia_with_NUP98_rearrangement</nowiki>. | <nowiki>*</nowiki>''Citation of this Page'': “Acute myeloid leukaemia with NUP98 rearrangement”. Compendium of Cancer Genome Aberrations (CCGA), Cancer Genomics Consortium (CGC), updated {{REVISIONMONTH}}/{{REVISIONDAY}}/{{REVISIONYEAR}}, <nowiki>https://ccga.io/index.php/HAEM5:Acute_myeloid_leukaemia_with_NUP98_rearrangement</nowiki>. | ||
| − | [[Category:HAEM5]][[Category:DISEASE]][[Category:Diseases A]] | + | [[Category:HAEM5]] |
| + | [[Category:DISEASE]] | ||
| + | [[Category:Diseases A]] | ||
| + | <references /> | ||
Latest revision as of 12:30, 24 March 2025
Haematolymphoid Tumours (WHO Classification, 5th ed.)
| This page is under construction |
(General Instructions – The focus of these pages is the clinically significant genetic alterations in each disease type. This is based on up-to-date knowledge from multiple resources such as PubMed and the WHO classification books. The CCGA is meant to be a supplemental resource to the WHO classification books; the CCGA captures in a continually updated wiki-stye manner the current genetics/genomics knowledge of each disease, which evolves more rapidly than books can be revised and published. If the same disease is described in multiple WHO classification books, the genetics-related information for that disease will be consolidated into a single main page that has this template (other pages would only contain a link to this main page). Use HUGO-approved gene names and symbols (italicized when appropriate), HGVS-based nomenclature for variants, as well as generic names of drugs and testing platforms or assays if applicable. Please complete tables whenever possible and do not delete them (add N/A if not applicable in the table and delete the examples); to add (or move) a row or column in a table, click nearby within the table and select the > symbol that appears. Please do not delete or alter the section headings. The use of bullet points alongside short blocks of text rather than only large paragraphs is encouraged. Additional instructions below in italicized blue text should not be included in the final page content. Please also see Author_Instructions and FAQs as well as contact your Associate Editor or Technical Support.)
Primary Author(s)*
Eric McGinnis, MD
Fatma Albulushi, MD
WHO Classification of Disease
| Structure | Disease |
|---|---|
| Book | Haematolymphoid Tumours (5th ed.) |
| Category | Myeloid proliferations and neoplasms |
| Family | Acute myeloid leukaemia |
| Type | Acute myeloid leukaemia with defining genetic abnormalities |
| Subtype(s) | Acute myeloid leukaemia with NUP98 rearrangement |
WHO Essential and Desirable Genetic Diagnostic Criteria.
| WHO Essential Criteria (Genetics)* | Detection of NUP98 rearrangement |
| WHO Desirable Criteria (Genetics)* | Identification of the NUP98 fusion partner |
| Other Classification | Myeloid blast count may <20% |
*Note: These are only the genetic/genomic criteria. Additional diagnostic criteria can be found in the WHO Classification of Tumours.
Related Terminology
(Instructions: The table will have the related terminology from the WHO autocompleted.)
| Acceptable | |
| Not Recommended |
Gene Rearrangements
Put your text here and fill in the table (Instructions: Details on clinical significance such as prognosis and other important information can be provided in the notes section. Please include references throughout the table. Do not delete the table.)
| Driver Gene | Fusion(s) and Common Partner Genes | Molecular Pathogenesis | Typical Chromosomal Alteration(s) | Prevalence -Common >20%, Recurrent 5-20% or Rare <5% (Disease) | Diagnostic, Prognostic, and Therapeutic Significance - D, P, T | Established Clinical Significance Per Guidelines - Yes or No (Source) | Clinical Relevance Details/Other Notes |
|---|---|---|---|---|---|---|---|
| EXAMPLE: ABL1 | EXAMPLE: BCR::ABL1 | EXAMPLE: The pathogenic derivative is the der(22) resulting in fusion of 5’ BCR and 3’ABL1. | EXAMPLE: t(9;22)(q34;q11.2) | EXAMPLE: Common (CML) | EXAMPLE: D, P, T | EXAMPLE: Yes (WHO, NCCN) | EXAMPLE:
The t(9;22) is diagnostic of CML in the appropriate morphology and clinical context (add reference). This fusion is responsive to targeted therapy such as Imatinib (Gleevec) (add reference). BCR::ABL1 is generally favorable in CML (add reference). |
| EXAMPLE: CIC | EXAMPLE: CIC::DUX4 | EXAMPLE: Typically, the last exon of CIC is fused to DUX4. The fusion breakpoint in CIC is usually intra-exonic and removes an inhibitory sequence, upregulating PEA3 genes downstream of CIC including ETV1, ETV4, and ETV5. | EXAMPLE: t(4;19)(q25;q13) | EXAMPLE: Common (CIC-rearranged sarcoma) | EXAMPLE: D | EXAMPLE:
DUX4 has many homologous genes; an alternate translocation in a minority of cases is t(10;19), but this is usually indistinguishable from t(4;19) by short-read sequencing (add references). | |
| EXAMPLE: ALK | EXAMPLE: ELM4::ALK
|
EXAMPLE: Fusions result in constitutive activation of the ALK tyrosine kinase. The most common ALK fusion is EML4::ALK, with breakpoints in intron 19 of ALK. At the transcript level, a variable (5’) partner gene is fused to 3’ ALK at exon 20. Rarely, ALK fusions contain exon 19 due to breakpoints in intron 18. | EXAMPLE: N/A | EXAMPLE: Rare (Lung adenocarcinoma) | EXAMPLE: T | EXAMPLE:
Both balanced and unbalanced forms are observed by FISH (add references). | |
| EXAMPLE: ABL1 | EXAMPLE: N/A | EXAMPLE: Intragenic deletion of exons 2–7 in EGFR removes the ligand-binding domain, resulting in a constitutively active tyrosine kinase with downstream activation of multiple oncogenic pathways. | EXAMPLE: N/A | EXAMPLE: Recurrent (IDH-wildtype Glioblastoma) | EXAMPLE: D, P, T | ||
Acute myeloid leukaemia (AML) with NUP98 rearrangement is characterized by chromosomal translocations involving NUP98 (nucleoporin 98 kDa) on chromosome 11p15.4 and various partner genes. (Reference WHO book). There are over 40 fusion partners which have been reported to date. NUP98 fusions can be categorized into three broad parts. The first category includes NUP98 fusions with transcription factors as partners, which can change the expression of target genes through DNA binding domains. The second category is NUP98 fusions with epigenetic modifiers that modify chromatin to change target gene expression. The third category of NUP98 fusions has neither the DNA binding nor chromatin remodeling domain.[1]
The NUP98 gene (chromosome 11p15) encodes a nucleoporin protein, which is part of the nuclear pore complex which regulates nucleocytoplasmic transport of protein and RNA. NUP98 fusion proteins involve the N-terminal portion of NUP98 and the C-terminal portion of the fusion partner. These fusion partners consist of homeodomain proteins, which are transcription factors, and non-homeodomain proteins, which are thought to play a role in transcriptional or epigenetic regulation.[1][2]
| Driver Gene | Fusion(s) and Common Partner Genes | Molecular Pathogenesis | Typical Chromosomal Alteration(s) | Prevalence -Common >20%, Recurrent 5-20% or Rare <5% (Disease) | Diagnostic, Prognostic, and Therapeutic Significance - D, P, T | Established Clinical Significance Per Guidelines - Yes or No (Source) | Clinical Relevance Details/Other Notes |
| NUP98 | NUP98::NSD1
|
NUP98-NSD1 prevents EZH2-mediated repression of Hox-A locus genes by colocalizing H3K36 methylation and histone acetylation at regulatory DNA elements hence preventing myeloid progenitor immortalization. | t(5;11)(q35;p15)
Usually cryptic |
Rare (AML) | Defining genetic abnormality in AML | Yes (WHO) | Rare but recurrent alteration seen mainly in children and young adults with AML. Poor overall survival, disease free survival, induction failure and chemotherapy resistance.[2] |
| NUP98 | NUP98::KDM5A | KDM5A is an epigenetic-modifying partners of NUP98 which dysregulate Hox genes expression through recognition of H3K4me3/2 marks by the plant homeodomain (PHD) finger domain. | t(11;12)(p15;p13)
Usually cryptic |
Rare (AML) | Defining genetic abnormality in AML | Yes (WHO) | Commonly associated with erythroid and megakaryocytic phenotypes in pediatric AML (acute erythroid leukemia and acute megakaryocytic leukemia).[2]
Usually associate with unfavorable outcomes |
| NUP98 | NUP98::HOXA9
|
NUP98 fusions bind near the HOX genes loci and activate their expression through chromatin remodeling. The overexpression of distal HoxA cluster genes promote self-renewal and drive leukogenesis. | t(7;11)(p15, p15) | Rare (AML) | Defining genetic abnormality in AML |
Individual Region Genomic Gain/Loss/LOH
Put your text here and fill in the table (Instructions: Includes aberrations not involving gene rearrangements. Details on clinical significance such as prognosis and other important information can be provided in the notes section. Can refer to CGC workgroup tables as linked on the homepage if applicable. Please include references throughout the table. Do not delete the table.)
| Chr # | Gain, Loss, Amp, LOH | Minimal Region Cytoband and/or Genomic Coordinates [Genome Build; Size] | Relevant Gene(s) | Diagnostic, Prognostic, and Therapeutic Significance - D, P, T | Established Clinical Significance Per Guidelines - Yes or No (Source) | Clinical Relevance Details/Other Notes |
|---|---|---|---|---|---|---|
| EXAMPLE:
7 |
EXAMPLE: Loss | EXAMPLE:
chr7 |
EXAMPLE:
Unknown |
EXAMPLE: D, P | EXAMPLE: No | EXAMPLE:
Presence of monosomy 7 (or 7q deletion) is sufficient for a diagnosis of AML with MDS-related changes when there is ≥20% blasts and no prior therapy (add reference). Monosomy 7/7q deletion is associated with a poor prognosis in AML (add references). |
| EXAMPLE:
8 |
EXAMPLE: Gain | EXAMPLE:
chr8 |
EXAMPLE:
Unknown |
EXAMPLE: D, P | EXAMPLE:
Common recurrent secondary finding for t(8;21) (add references). | |
| EXAMPLE:
17 |
EXAMPLE: Amp | EXAMPLE:
17q12; chr17:39,700,064-39,728,658 [hg38; 28.6 kb] |
EXAMPLE:
ERBB2 |
EXAMPLE: D, P, T | EXAMPLE:
Amplification of ERBB2 is associated with HER2 overexpression in HER2 positive breast cancer (add references). Add criteria for how amplification is defined. | |
No characteristic chromosomal gain or loss. However, trisomy 8 and chromosome 13 abnormalities may be observed.
Several reports indicated that del(13q) is a frequent event in NUP98::KDM5A AML patients, indicating co-occurrence of NUP98-KDMA fusion with RB1 deletion.
| Chromosome Number | Gain/Loss/Amp/LOH | Minimal Region Cytoband and/or Genomic Coordinates [Genome Build; Size] | Relevant Gene(s) | Diagnostic, Prognostic, and Therapeutic Significance - D, P, T | Established Clinical Significance Per Guidelines - Yes or No (Source) | Clinical Relevance Details/Other Notes |
| 8 | Gain | Trisomy 8 | Unknown | NA | No | |
| 13 | loss | Deletion of 13q | RB1 gene | NA | Particularly associated with NUP98::KDM5A |
Characteristic Chromosomal or Other Global Mutational Patterns
Put your text here and fill in the table (Instructions: Included in this category are alterations such as hyperdiploid; gain of odd number chromosomes including typically chromosome 1, 3, 5, 7, 11, and 17; co-deletion of 1p and 19q; complex karyotypes without characteristic genetic findings; chromothripsis; microsatellite instability; homologous recombination deficiency; mutational signature pattern; etc. Details on clinical significance such as prognosis and other important information can be provided in the notes section. Please include references throughout the table. Do not delete the table.)
| Chromosomal Pattern | Molecular Pathogenesis | Prevalence -
Common >20%, Recurrent 5-20% or Rare <5% (Disease) |
Diagnostic, Prognostic, and Therapeutic Significance - D, P, T | Established Clinical Significance Per Guidelines - Yes or No (Source) | Clinical Relevance Details/Other Notes |
|---|---|---|---|---|---|
| EXAMPLE:
Co-deletion of 1p and 18q |
EXAMPLE: See chromosomal rearrangements table as this pattern is due to an unbalanced derivative translocation associated with oligodendroglioma (add reference). | EXAMPLE: Common (Oligodendroglioma) | EXAMPLE: D, P | ||
| EXAMPLE:
Microsatellite instability - hypermutated |
EXAMPLE: Common (Endometrial carcinoma) | EXAMPLE: P, T | |||
Gene Mutations (SNV/INDEL)
Put your text here and fill in the table (Instructions: This table is not meant to be an exhaustive list; please include only genes/alterations that are recurrent or common as well either disease defining and/or clinically significant. If a gene has multiple mechanisms depending on the type or site of the alteration, add multiple entries in the table. For clinical significance, denote associations with FDA-approved therapy (not an extensive list of applicable drugs) and NCCN or other national guidelines if applicable; Can also refer to CGC workgroup tables as linked on the homepage if applicable as well as any high impact papers or reviews of gene mutations in this entity. Details on clinical significance such as prognosis and other important information such as concomitant and mutually exclusive mutations can be provided in the notes section. Please include references throughout the table. Do not delete the table.)
| Gene | Genetic Alteration | Tumor Suppressor Gene, Oncogene, Other | Prevalence -
Common >20%, Recurrent 5-20% or Rare <5% (Disease) |
Diagnostic, Prognostic, and Therapeutic Significance - D, P, T | Established Clinical Significance Per Guidelines - Yes or No (Source) | Clinical Relevance Details/Other Notes |
|---|---|---|---|---|---|---|
| EXAMPLE:EGFR
|
EXAMPLE: Exon 18-21 activating mutations | EXAMPLE: Oncogene | EXAMPLE: Common (lung cancer) | EXAMPLE: T | EXAMPLE: Yes (NCCN) | EXAMPLE: Exons 18, 19, and 21 mutations are targetable for therapy. Exon 20 T790M variants cause resistance to first generation TKI therapy and are targetable by second and third generation TKIs (add references). |
| EXAMPLE: TP53; Variable LOF mutations
|
EXAMPLE: Variable LOF mutations | EXAMPLE: Tumor Supressor Gene | EXAMPLE: Common (breast cancer) | EXAMPLE: P | EXAMPLE: >90% are somatic; rare germline alterations associated with Li-Fraumeni syndrome (add reference). Denotes a poor prognosis in breast cancer. | |
| EXAMPLE: BRAF; Activating mutations | EXAMPLE: Activating mutations | EXAMPLE: Oncogene | EXAMPLE: Common (melanoma) | EXAMPLE: T | ||
Note: A more extensive list of mutations can be found in cBioportal, COSMIC, and/or other databases. When applicable, gene-specific pages within the CCGA site directly link to pertinent external content.
FLT3-ITD and WT1 mutation are recurring events in NUP98::NSD1 and was also observed in some NUP98::HOXA9 AML patients.(R1). Loss of RB1 at 13q14 is particularly associated with NUP98::KDM5A
| Gene | Genetic Alteration | Tumor Suppressor Gene (TSG)/Oncogene/Other | Prevalence -Common >20%, Recurrent 5-20% or Rare <5% (Disease) | Diagnostic, Prognostic, and Therapeutic Significance - D, P, T | Established Clinical Significance Per Guidelines - Yes or No (Source) | Clinical Relevance Details/Other Notes |
| FLT3-ITD | Recurrent | Poor prognosis | Seen in 67 to 91% of cases with NUP98::NSD1 | |||
| WT1 | Rare | Reported in 33-55% of NUP98::NSD1 rearranged AML | ||||
| RB1 | Rare | Particularly associated with NUP98::KDM5A |
Epigenomic Alterations
Put your text here
Genes and Main Pathways Involved
Put your text here and fill in the table (Instructions: Please include references throughout the table. Do not delete the table.)
| Gene; Genetic Alteration | Pathway | Pathophysiologic Outcome |
|---|---|---|
| EXAMPLE: BRAF and MAP2K1; Activating mutations | EXAMPLE: MAPK signaling | EXAMPLE: Increased cell growth and proliferation |
| EXAMPLE: CDKN2A; Inactivating mutations | EXAMPLE: Cell cycle regulation | EXAMPLE: Unregulated cell division |
| EXAMPLE: KMT2C and ARID1A; Inactivating mutations | EXAMPLE: Histone modification, chromatin remodeling | EXAMPLE: Abnormal gene expression program |
Genetic Diagnostic Testing Methods
Rearrangements involving NUP98 are often cryptic on conventional karyotype, owing to terminal location of NUP98 on chromosome 11p15.4. Most patients have a normal karyotype. Diagnosis is established using the following tests:
- FISH using NUP98 break-apart probes
- RT-PCR for fusion proteins like NUP98::NSD1
- RNA sequencing
- Optical Genome Mapping (OGM)
Familial Forms
Put your text here (Instructions: Include associated hereditary conditions/syndromes that cause this entity or are caused by this entity.)
Additional Information
Links
Put a link here or anywhere appropriate in this page (Instructions: Highlight the text to which you want to add a link in this section or elsewhere, select the "Link" icon at the top of the wiki page, and search the name of the internal page to which you want to link this text, or enter an external internet address by including the "http://www." portion.)
References
(use the "Cite" icon at the top of the page) (Instructions: Add each reference into the text above by clicking where you want to insert the reference, selecting the “Cite” icon at the top of the wiki page, and using the “Automatic” tab option to search by PMID to select the reference to insert. If a PMID is not available, such as for a book, please use the “Cite” icon, select “Manual” and then “Basic Form”, and include the entire reference. To insert the same reference again later in the page, select the “Cite” icon and “Re-use” to find the reference; DO NOT insert the same reference twice using the “Automatic” tab as it will be treated as two separate references. The reference list in this section will be automatically generated and sorted.)
Notes
*Primary authors will typically be those that initially create and complete the content of a page. If a subsequent user modifies the content and feels the effort put forth is of high enough significance to warrant listing in the authorship section, please contact the Associate Editor or other CCGA representative. When pages have a major update, the new author will be acknowledged at the beginning of the page, and those who contributed previously will be acknowledged below as a prior author.
Prior Author(s):
*Citation of this Page: “Acute myeloid leukaemia with NUP98 rearrangement”. Compendium of Cancer Genome Aberrations (CCGA), Cancer Genomics Consortium (CGC), updated 03/24/2025, https://ccga.io/index.php/HAEM5:Acute_myeloid_leukaemia_with_NUP98_rearrangement.
- ↑ Jump up to: 1.0 1.1 Mohanty, Sagarajit (2023-09). "NUP98 Rearrangements in AML: Molecular Mechanisms and Clinical Implications". Onco. 3 (3): 147–164. doi:10.3390/onco3030011. ISSN 2673-7523. Check date values in:
|date=(help) - ↑ Jump up to: 2.0 2.1 2.2 Bertrums, Eline J. M.; et al. (2023-02-23). "Comprehensive molecular and clinical characterization of NUP98 fusions in pediatric acute myeloid leukemia". Haematologica. 108 (8): 2044–2058. doi:10.3324/haematol.2022.281653. ISSN 1592-8721.