Difference between revisions of "Testpage"
| [unchecked revision] | [unchecked revision] |
| Line 13: | Line 13: | ||
==Cancer Sub-Classification / Subtype== | ==Cancer Sub-Classification / Subtype== | ||
| − | Acute myeloid leukemia (AML) with t(6;9)(p23;q34.1) resulting in DEK-NUP214 fusion. | + | Acute myeloid leukemia (AML) with t(6;9)(p23;q34.1) resulting in DEK-NUP214 fusion.1 |
==Definition / Description of Disease<ref>{{Cite journal|date=2004 Sep|title=Acute myeloid leukemia with t(6;9)(p23;q34) is associated with dysplasia and a high frequency of flt3 gene mutations|url=https://pubmed.ncbi.nlm.nih.gov/15362364/|journal=American journal of clinical pathology|language=en|volume=122|issue=3|doi=10.1309/5DGB-59KQ-A527-PD47|issn=0002-9173}}</ref>== | ==Definition / Description of Disease<ref>{{Cite journal|date=2004 Sep|title=Acute myeloid leukemia with t(6;9)(p23;q34) is associated with dysplasia and a high frequency of flt3 gene mutations|url=https://pubmed.ncbi.nlm.nih.gov/15362364/|journal=American journal of clinical pathology|language=en|volume=122|issue=3|doi=10.1309/5DGB-59KQ-A527-PD47|issn=0002-9173}}</ref>== | ||
Revision as of 17:22, 11 December 2020
Primary Author(s)*
Jennelle C. Hodge, PhD, FACMG
Cancer Category/Type
Cancer Sub-Classification / Subtype
Acute myeloid leukemia (AML) with t(6;9)(p23;q34.1) resulting in DEK-NUP214 fusion.1
Definition / Description of Disease[1]
This is a distinct entity in the World Health Organization (WHO) classification system, and the most common associated French-American-British (FAB) classifications are M2, M4 and M1.[2][3]
Synonyms / Terminology
None
Epidemiology / Prevalence
Accounts for 0.7-1.8% of AML, occurring in both children (median age of 13 years) and adults (median age of 35-44 years).[2]
Clinical Features
Usually presents with anemia and thrombocytopenia and often with pancytopenia. In adults, the median white blood cell count is 12x10^9/L, which is generally lower than other AML types.[2]
Sites of Involvement
Bone marrow
Morphologic Features
This AML subtype may present with or without monocytic features and is frequently associated with basophilia (44-62% of cases) and multilineage dysplasia (most commonly granulocytic and erythroid, and less often megakaryocytic dysplasia).[2] Auer rods occur in ~1/3 of cases and ringed sideroblasts may also occur.[2] The peripheral blood or bone marrow have >20% blasts characterized by a non-specific myeloid immunophenotype.[2]
Immunophenotype
The characteristic immunophenotype of the blasts associated with this entity is listed in the table below. In addition, basophils may present as separate clusters of CD123+, CD33+ CD38+ and HLA-DR- cells.[2]
| Finding | Marker |
|---|---|
| Positive (universal) | Myeloperoxidase (MPO), CD9, CD13, CD33, CD38 CD123, and HLA-DR |
| Positive (subset) | KIT(CD117), CD34, CD15, CD64 (monocyte-associated marker), and Dexoynucleotidyl Transferase (TdT) |
| Negative (universal) | |
| Negative (subset) |
Chromosomal Rearrangements (Gene Fusions)
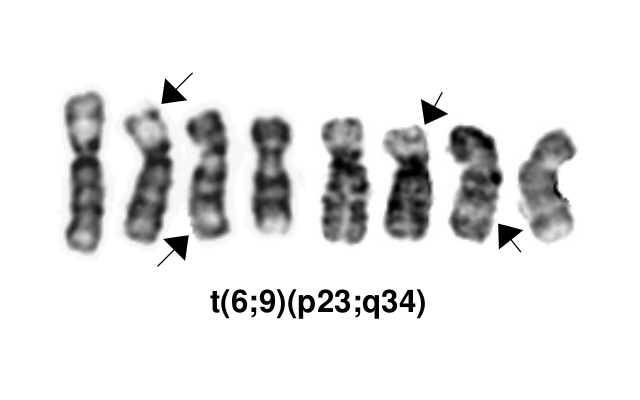
This AML subtype is classified based on the presence of a t(6;9)(p23;q34.1), which results in fusion of the 5’ portion of DEK at “6p23” (specifically 6p22.3[hg38]) and the 3’ portion of NUP214(CAN) at “9q34.1” (specifically 9q34.13[hg38]). The breakpoints are intronic, producing an in-frame fusion.[4] The DEK-NUP214 fusion present on the derivative chromosome 6 is considered the pathogenic entity as the reciprocal NUP214-DEK fusion on chromosome 9 does not appear to be transcribed.[5] Typically the DEK-NUP214 fusion presents as the sole abnormality but can be part of a complex karyotype.[2]
| Chromosomal Rearrangement | Genes in Fusion (5’ or 3’ Segments) | Pathogenic Derivative | Prevalence |
|---|---|---|---|
| t(6;9)(p23;q34.1) | 5'DEK / 3'NUP214(CAN) | der(6) | 0.7-1.8% of AML |
Characteristic Chromosomal Aberrations / Patterns
Not applicable
Genomic Gain/Loss/LOH
Not applicable
Gene Mutations (SNV/INDEL)
COSMIC does not have specific information on mutations related to this subtype of AML.
Other Mutations
| Type | Gene/Region/Other |
|---|---|
| Concomitant Mutations | FLT3-ITD (69% of children and 78% of adults) |
| Secondary Mutations | |
| Mutually Exclusive | FLT3-TKD is very uncommon |
Epigenomics (Methylation)
Not applicable
Genes and Main Pathways Involved
The molecular mechanism is not completely understand, but the fusion protein is known to act as an aberrant transcription factor, alter nuclear transport and induce myeloid cell-specific global protein synthesis.[2][6]
Diagnostic Testing Methods
Karyotype, FISH, RT-PCR
Clinical Significance (Diagnosis, Prognosis and Therapeutic Implications)
This translocation has traditionally been associated with a poor prognosis in both adult and pediatric cases.[2] Of note, a 2014 retrospective analysis suggests a better outcome for pediatric patients with this translocation than previously reported.[7] Elevated white blood cell counts and higher bone marrow blast percentages are associated with shorter periods of overall survival and disease-free survival, respectively.[2]
Limited data suggests early allogeneic stem cell transplantation may be associated with better overall survival compared to patients without transplantation, suggesting accurate diagnosis for these patients is crucial.[2][8][9]
The concurrent presence of FLT3-ITD does not appear to negatively impact survival in the pediatric population.[2]
Cases with the 6;9 translocation and <20% blasts are not currently classified as AML, which is controversial. Such cases should have close follow-up to monitor for development of more definitive evidence of AML or may be treated as AML if clinically appropriate.[2]
Familial Forms
Not applicable
Other Information
Not applicable
Links
References
- ↑ "Acute myeloid leukemia with t(6;9)(p23;q34) is associated with dysplasia and a high frequency of flt3 gene mutations". American journal of clinical pathology. 122 (3). 2004 Sep. doi:10.1309/5DGB-59KQ-A527-PD47. ISSN 0002-9173. Check date values in:
|date=(help) - ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 Arber DA, et al., (2017). Acute myeloid leukaemia with recurrent genetic abnormalities, in World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues, Revised 4th edition. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Arber DA, Hasserjian RP, Le Beau MM, Orazi A, and Siebert R, Editors. IARC Press: Lyon, France, p137-138.
- ↑ Oyarzo, Mauricio P.; et al. (2004). "Acute myeloid leukemia with t(6;9)(p23;q34) is associated with dysplasia and a high frequency of flt3 gene mutations". American Journal of Clinical Pathology. 122 (3): 348–358. doi:10.1309/5DGB-59KQ-A527-PD47. ISSN 0002-9173. PMID 15362364.
- ↑ von Lindern, M.; et al. (1992). "The translocation (6;9), associated with a specific subtype of acute myeloid leukemia, results in the fusion of two genes, dek and can, and the expression of a chimeric, leukemia-specific dek-can mRNA". Molecular and Cellular Biology. 12 (4): 1687–1697. doi:10.1128/mcb.12.4.1687. ISSN 0270-7306. PMC 369612. PMID 1549122.CS1 maint: PMC format (link)
- ↑ von Lindern, M.; et al. (1992). "Translocation t(6;9) in acute non-lymphocytic leukaemia results in the formation of a DEK-CAN fusion gene". Bailliere's Clinical Haematology. 5 (4): 857–879. ISSN 0950-3536. PMID 1308167.
- ↑ Ageberg, Malin; et al. (2008). "Identification of a novel and myeloid specific role of the leukemia-associated fusion protein DEK-NUP214 leading to increased protein synthesis". Genes, Chromosomes & Cancer. 47 (4): 276–287. doi:10.1002/gcc.20531. ISSN 1098-2264. PMID 18181180.
- ↑ Sandahl, Julie Damgaard; et al. (2014). "t(6;9)(p22;q34)/DEK-NUP214-rearranged pediatric myeloid leukemia: an international study of 62 patients". Haematologica. 99 (5): 865–872. doi:10.3324/haematol.2013.098517. ISSN 1592-8721. PMC 4008104. PMID 24441146.
- ↑ Slovak, M. L.; et al. (2006). "A retrospective study of 69 patients with t(6;9)(p23;q34) AML emphasizes the need for a prospective, multicenter initiative for rare 'poor prognosis' myeloid malignancies". Leukemia. 20 (7): 1295–1297. doi:10.1038/sj.leu.2404233. ISSN 0887-6924. PMID 16628187.
- ↑ Ishiyama, K.; et al. (2012). "Allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with t(6;9)(p23;q34) dramatically improves the patient prognosis: a matched-pair analysis". Leukemia. 26 (3): 461–464. doi:10.1038/leu.2011.229. ISSN 1476-5551. PMID 21869835.
Notes
*Primary authors will typically be those that initially create and complete the content of a page. If a subsequent user modifies the content and feels the effort put forth is of high enough significance to warrant listing in the authorship section, please contact the CCGA coordinators (contact information provided on the homepage). Additional global feedback or concerns are also welcome.