Difference between revisions of "Giant cell glioblastoma"
Jump to navigation
Jump to search
| [unchecked revision] | [unchecked revision] |
| (One intermediate revision by the same user not shown) | |||
| Line 8: | Line 8: | ||
==Cancer Category/Type== | ==Cancer Category/Type== | ||
| − | * Diffuse astrocytic and olidodendroglial tumors | + | *Diffuse astrocytic and olidodendroglial tumors |
==Cancer Sub-Classification / Subtype== | ==Cancer Sub-Classification / Subtype== | ||
| − | Glioblastoma, IDH-wildtype (IDH-wt) | + | [[Glioblastoma, IDH-wildtype (IDH-wt)]] |
==Definition / Description of Disease== | ==Definition / Description of Disease== | ||
| − | * Rare histologic variant of IDH-wt glioblastoma <ref name=":0">WHO Classification of Tumours of the Central Nervous System, 4th ed, Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (Eds), IARC, Lyon 2016. Pg 46-47</ref> | + | *Rare histologic variant of IDH-wt glioblastoma <ref name=":0">WHO Classification of Tumours of the Central Nervous System, 4th ed, Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (Eds), IARC, Lyon 2016. Pg 46-47</ref> |
| − | * Large, multinucleate giant cells with occasional abundant reticuln network <ref name=":0" /> | + | *Large, multinucleate giant cells with occasional abundant reticuln network <ref name=":0" /> |
==Synonyms / Terminology== | ==Synonyms / Terminology== | ||
| Line 23: | Line 23: | ||
==Epidemiology / Prevalence== | ==Epidemiology / Prevalence== | ||
| − | * Constitute <1% of glioblastomas <ref name=":1">{{Cite journal|last=Ortega|first=Alicia|last2=Nuño|first2=Miriam|last3=Walia|first3=Sartaaj|last4=Mukherjee|first4=Debraj|last5=Black|first5=Keith L.|last6=Patil|first6=Chirag G.|date=2014|title=Treatment and survival of patients harboring histological variants of glioblastoma|url=https://www.ncbi.nlm.nih.gov/pubmed/24980627|journal=Journal of Clinical Neuroscience: Official Journal of the Neurosurgical Society of Australasia|volume=21|issue=10|pages=1709–1713|doi=10.1016/j.jocn.2014.05.003|issn=1532-2653|pmid=24980627}}</ref> | + | *Constitute <1% of glioblastomas <ref name=":1">{{Cite journal|last=Ortega|first=Alicia|last2=Nuño|first2=Miriam|last3=Walia|first3=Sartaaj|last4=Mukherjee|first4=Debraj|last5=Black|first5=Keith L.|last6=Patil|first6=Chirag G.|date=2014|title=Treatment and survival of patients harboring histological variants of glioblastoma|url=https://www.ncbi.nlm.nih.gov/pubmed/24980627|journal=Journal of Clinical Neuroscience: Official Journal of the Neurosurgical Society of Australasia|volume=21|issue=10|pages=1709–1713|doi=10.1016/j.jocn.2014.05.003|issn=1532-2653|pmid=24980627}}</ref> |
| − | * May be more common in pediatric population <ref name=":2">{{Cite journal|last=Kozak|first=Kevin R.|last2=Moody|first2=John S.|date=2009|title=Giant cell glioblastoma: a glioblastoma subtype with distinct epidemiology and superior prognosis|url=https://www.ncbi.nlm.nih.gov/pubmed/19332771|journal=Neuro-Oncology|volume=11|issue=6|pages=833–841|doi=10.1215/15228517-2008-123|issn=1523-5866|pmc=2802403|pmid=19332771}}</ref> | + | *May be more common in pediatric population <ref name=":2">{{Cite journal|last=Kozak|first=Kevin R.|last2=Moody|first2=John S.|date=2009|title=Giant cell glioblastoma: a glioblastoma subtype with distinct epidemiology and superior prognosis|url=https://www.ncbi.nlm.nih.gov/pubmed/19332771|journal=Neuro-Oncology|volume=11|issue=6|pages=833–841|doi=10.1215/15228517-2008-123|issn=1523-5866|pmc=2802403|pmid=19332771}}</ref> |
| − | * Mean age 51 years <ref name=":2" /> | + | *Mean age 51 years <ref name=":2" /> |
==Clinical Features== | ==Clinical Features== | ||
| − | * No evidence of a precursor lesion <ref name=":0" /> | + | *No evidence of a precursor lesion <ref name=":0" /> |
| − | * Presenting symptoms similar to IDH-wt glioblastoma | + | *Presenting symptoms similar to IDH-wt glioblastoma |
| − | * Radiographically and grossly circumscribed borders, may be mistaken for other neoplastic or non-neoplastic lesions <ref>{{Cite journal|last=Turner|first=Ryan|last2=Matthys|first2=Samuel|last3=Heymann|first3=John|last4=Gelman|first4=Benjamin|date=2018|title=Imaging findings in the progression of a giant cell glioblastoma|url=https://www.ncbi.nlm.nih.gov/pubmed/30128062|journal=Radiology Case Reports|volume=13|issue=5|pages=1007–1011|doi=10.1016/j.radcr.2018.07.010|issn=1930-0433|pmc=6097408|pmid=30128062}}</ref> | + | *Radiographically and grossly circumscribed borders, may be mistaken for other neoplastic or non-neoplastic lesions <ref name=":6">{{Cite journal|last=Turner|first=Ryan|last2=Matthys|first2=Samuel|last3=Heymann|first3=John|last4=Gelman|first4=Benjamin|date=2018|title=Imaging findings in the progression of a giant cell glioblastoma|url=https://www.ncbi.nlm.nih.gov/pubmed/30128062|journal=Radiology Case Reports|volume=13|issue=5|pages=1007–1011|doi=10.1016/j.radcr.2018.07.010|issn=1930-0433|pmc=6097408|pmid=30128062}}</ref> |
==Sites of Involvement== | ==Sites of Involvement== | ||
| − | * Localization similar to IDH-wt glioblastoma <ref name=":2" /><ref name=":1" /> | + | *Localization similar to IDH-wt glioblastoma <ref name=":2" /><ref name=":1" /> |
==Morphologic Features== | ==Morphologic Features== | ||
| − | * Bizarre, multi-nucleate giant cells with atypical mitotic figures <ref>{{Cite journal|last=Margetts|first=J. C.|last2=Kalyan-Raman|first2=U. P.|date=1989|title=Giant-celled glioblastoma of brain. A clinico-pathological and radiological study of ten cases (including immunohistochemistry and ultrastructure)|url=https://www.ncbi.nlm.nih.gov/pubmed/2912529|journal=Cancer|volume=63|issue=3|pages=524–531|doi=10.1002/1097-0142(19890201)63:33.0.co;2-d|issn=0008-543X|pmid=2912529}}</ref> | + | *Bizarre, multi-nucleate giant cells with atypical mitotic figures <ref>{{Cite journal|last=Margetts|first=J. C.|last2=Kalyan-Raman|first2=U. P.|date=1989|title=Giant-celled glioblastoma of brain. A clinico-pathological and radiological study of ten cases (including immunohistochemistry and ultrastructure)|url=https://www.ncbi.nlm.nih.gov/pubmed/2912529|journal=Cancer|volume=63|issue=3|pages=524–531|doi=10.1002/1097-0142(19890201)63:33.0.co;2-d|issn=0008-543X|pmid=2912529}}</ref> |
| − | * Cells may contain numerous nuclei <ref name=":0" /> | + | *Cells may contain numerous nuclei <ref name=":0" /> |
| − | * Palisading and ischemic necrosis <ref name=":0" /> | + | *Palisading and ischemic necrosis <ref name=":0" /> |
| − | * Pseudo-rosette like perivascular tumor cell concentration <ref name=":0" /> | + | *Pseudo-rosette like perivascular tumor cell concentration <ref name=":0" /> |
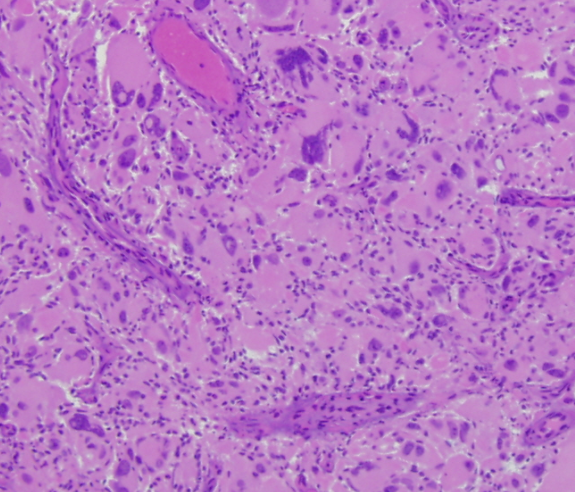
[[File:GCG sample image.png|thumb|Hematoxylin and Eosin stained section of giant cell glioblastoma showing bizarre multinucleate cells]] | [[File:GCG sample image.png|thumb|Hematoxylin and Eosin stained section of giant cell glioblastoma showing bizarre multinucleate cells]] | ||
| Line 51: | Line 51: | ||
|- | |- | ||
!Finding!!Marker | !Finding!!Marker | ||
| + | |- | ||
| + | |Positive (by definition) | ||
| + | |IDH-1 | ||
|- | |- | ||
|Positive (variable level)||GFAP <ref name=":3">{{Cite journal|last=Katoh|first=M.|last2=Aida|first2=T.|last3=Sugimoto|first3=S.|last4=Suwamura|first4=Y.|last5=Abe|first5=H.|last6=Isu|first6=T.|last7=Kaneko|first7=S.|last8=Mitsumori|first8=K.|last9=Kojima|first9=H.|date=1995|title=Immunohistochemical analysis of giant cell glioblastoma|url=https://www.ncbi.nlm.nih.gov/pubmed/7550996|journal=Pathology International|volume=45|issue=4|pages=275–282|doi=10.1111/j.1440-1827.1995.tb03456.x|issn=1320-5463|pmid=7550996}}</ref> | |Positive (variable level)||GFAP <ref name=":3">{{Cite journal|last=Katoh|first=M.|last2=Aida|first2=T.|last3=Sugimoto|first3=S.|last4=Suwamura|first4=Y.|last5=Abe|first5=H.|last6=Isu|first6=T.|last7=Kaneko|first7=S.|last8=Mitsumori|first8=K.|last9=Kojima|first9=H.|date=1995|title=Immunohistochemical analysis of giant cell glioblastoma|url=https://www.ncbi.nlm.nih.gov/pubmed/7550996|journal=Pathology International|volume=45|issue=4|pages=275–282|doi=10.1111/j.1440-1827.1995.tb03456.x|issn=1320-5463|pmid=7550996}}</ref> | ||
|- | |- | ||
| − | |Positive ( | + | |Positive (Majority)||P53 <ref>{{Cite journal|last=Peraud|first=A.|last2=Watanabe|first2=K.|last3=Plate|first3=K. H.|last4=Yonekawa|first4=Y.|last5=Kleihues|first5=P.|last6=Ohgaki|first6=H.|date=1997|title=p53 mutations versus EGF receptor expression in giant cell glioblastomas|url=https://www.ncbi.nlm.nih.gov/pubmed/9370234|journal=Journal of Neuropathology and Experimental Neurology|volume=56|issue=11|pages=1236–1241|doi=10.1097/00005072-199711000-00008|issn=0022-3069|pmid=9370234}}</ref> <ref name=":3" /> |
|- | |- | ||
| − | |Negative (universal)|| | + | |Negative (nearly universal)||Neuronal Antigens <ref name=":7">{{Cite journal|last=Martinez-Diaz|first=Hilda|last2=Kleinschmidt-DeMasters|first2=B. K.|last3=Powell|first3=Suzanne Z.|last4=Yachnis|first4=Anthony T.|date=2003|title=Giant cell glioblastoma and pleomorphic xanthoastrocytoma show different immunohistochemical profiles for neuronal antigens and p53 but share reactivity for class III beta-tubulin|url=https://www.ncbi.nlm.nih.gov/pubmed/12946225|journal=Archives of Pathology & Laboratory Medicine|volume=127|issue=9|pages=1187–1191|doi=10.1043/1543-2165(2003)1272.0.CO;2|issn=1543-2165|pmid=12946225}}</ref> |
|} | |} | ||
| Line 78: | Line 81: | ||
==Genomic Gain/Loss/LOH== | ==Genomic Gain/Loss/LOH== | ||
| − | + | *Near haploidization may be a primary oncogenic event underlying a subset of GCG's <ref>{{Cite journal|last=Bigner|first=S. H.|last2=Mark|first2=J.|last3=Schold|first3=S. C.|last4=Eng|first4=L. F.|last5=Bigner|first5=D. D.|date=1985|title=A serially transplantable human giant cell glioblastoma that maintains a near-haploid stem line|url=https://www.ncbi.nlm.nih.gov/pubmed/3840409|journal=Cancer Genetics and Cytogenetics|volume=18|issue=2|pages=141–153|doi=10.1016/0165-4608(85)90064-0|issn=0165-4608|pmid=3840409}}</ref> | |
| + | **Recurrent loss of chromosomes 1, 2, 3, 4, 5, 6, 8, 9, 10, 11, 13, 14, 15, 17, 18, 19, 22 | ||
| + | **Typically have gains or retain heterozygosity of chromosome 7 | ||
| + | *Similar genomic findings have been described in other oncocytic tumors <ref name=":4">{{Cite journal|last=Ganly|first=Ian|last2=Makarov|first2=Vladimir|last3=Deraje|first3=Shyamprasad|last4=Dong|first4=YiYu|last5=Reznik|first5=Ed|last6=Seshan|first6=Venkatraman|last7=Nanjangud|first7=Gouri|last8=Eng|first8=Stephanie|last9=Bose|first9=Promita|date=2018|title=Integrated Genomic Analysis of Hürthle Cell Cancer Reveals Oncogenic Drivers, Recurrent Mitochondrial Mutations, and Unique Chromosomal Landscapes|url=https://www.ncbi.nlm.nih.gov/pubmed/30107176|journal=Cancer Cell|volume=34|issue=2|pages=256–270.e5|doi=10.1016/j.ccell.2018.07.002|issn=1878-3686|pmc=6247912|pmid=30107176}}</ref> <ref>{{Cite journal|last=Corver|first=Willem E.|last2=Ruano|first2=Dina|last3=Weijers|first3=Karin|last4=den Hartog|first4=Wietske C. E.|last5=van Nieuwenhuizen|first5=Merlijn P.|last6=de Miranda|first6=Noel|last7=van Eijk|first7=Ronald|last8=Middeldorp|first8=Anneke|last9=Jordanova|first9=Ekaterina S.|date=2012|title=Genome haploidisation with chromosome 7 retention in oncocytic follicular thyroid carcinoma|url=https://www.ncbi.nlm.nih.gov/pubmed/22675538|journal=PloS One|volume=7|issue=6|pages=e38287|doi=10.1371/journal.pone.0038287|issn=1932-6203|pmc=3365880|pmid=22675538}}</ref> <ref>{{Cite journal|last=Corver|first=Willem E.|last2=van Wezel|first2=Tom|last3=Molenaar|first3=Kees|last4=Schrumpf|first4=Melanie|last5=van den Akker|first5=Brendy|last6=van Eijk|first6=Ronald|last7=Ruano Neto|first7=Dina|last8=Oosting|first8=Jan|last9=Morreau|first9=Hans|date=2014|title=Near-haploidization significantly associates with oncocytic adrenocortical, thyroid, and parathyroid tumors but not with mitochondrial DNA mutations|url=https://www.ncbi.nlm.nih.gov/pubmed/24909752|journal=Genes, Chromosomes & Cancer|volume=53|issue=10|pages=833–844|doi=10.1002/gcc.22194|issn=1098-2264|pmid=24909752}}</ref> <ref>{{Cite journal|last=Gopal|first=Raj K.|last2=Kübler|first2=Kirsten|last3=Calvo|first3=Sarah E.|last4=Polak|first4=Paz|last5=Livitz|first5=Dimitri|last6=Rosebrock|first6=Daniel|last7=Sadow|first7=Peter M.|last8=Campbell|first8=Braidie|last9=Donovan|first9=Samuel E.|date=2018|title=Widespread Chromosomal Losses and Mitochondrial DNA Alterations as Genetic Drivers in Hürthle Cell Carcinoma|url=https://www.ncbi.nlm.nih.gov/pubmed/30107175|journal=Cancer Cell|volume=34|issue=2|pages=242–255.e5|doi=10.1016/j.ccell.2018.06.013|issn=1878-3686|pmc=6121811|pmid=30107175}}</ref> | ||
| + | *Oncogenenesis in such cases may be related to widespread loss of tumor suppressors in a catastrophic reduplication event early in tumorigenesis <ref name=":4" /> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==Gene Mutations (SNV/INDEL)== | ==Gene Mutations (SNV/INDEL)== | ||
| Line 97: | Line 95: | ||
!Gene!!Mutation!!Oncogene/Tumor Suppressor/Other!!Presumed Mechanism (LOF/GOF/Other; Driver/Passenger)!!Prevalence (COSMIC/TCGA/Other) | !Gene!!Mutation!!Oncogene/Tumor Suppressor/Other!!Presumed Mechanism (LOF/GOF/Other; Driver/Passenger)!!Prevalence (COSMIC/TCGA/Other) | ||
|- | |- | ||
| − | | | + | |TP53|| ||Tumor Suppressor|| ||75-90% <ref>{{Cite journal|last=Martinez|first=Ramon|last2=Roggendorf|first2=Wolfgang|last3=Baretton|first3=Gustavo|last4=Klein|first4=Rüdiger|last5=Toedt|first5=Grisha|last6=Lichter|first6=Peter|last7=Schackert|first7=Gabriele|last8=Joos|first8=Stefan|date=2007|title=Cytogenetic and molecular genetic analyses of giant cell glioblastoma multiforme reveal distinct profiles in giant cell and non-giant cell subpopulations|url=https://www.ncbi.nlm.nih.gov/pubmed/17498554|journal=Cancer Genetics and Cytogenetics|volume=175|issue=1|pages=26–34|doi=10.1016/j.cancergencyto.2007.01.006|issn=0165-4608|pmid=17498554}}</ref> |
| + | |- | ||
| + | |PTEN | ||
| + | | | ||
| + | |Tumor Suppressor | ||
| + | | | ||
| + | |33% <ref name=":5">{{Cite journal|last=Oh|first=Ji Eun|last2=Ohta|first2=Takashi|last3=Nonoguchi|first3=Naosuke|last4=Satomi|first4=Kaishi|last5=Capper|first5=David|last6=Pierscianek|first6=Daniela|last7=Sure|first7=Ulrich|last8=Vital|first8=Anne|last9=Paulus|first9=Werner|date=2016|title=Genetic Alterations in Gliosarcoma and Giant Cell Glioblastoma|url=https://www.ncbi.nlm.nih.gov/pubmed/26443480|journal=Brain Pathology (Zurich, Switzerland)|volume=26|issue=4|pages=517–522|doi=10.1111/bpa.12328|issn=1750-3639|pmid=26443480}}</ref> | ||
| + | |- | ||
| + | |ATRX | ||
| + | | | ||
| + | |Tumor Suppressor | ||
| + | |Expression loss | ||
| + | |19% <ref name=":5" /> | ||
| + | |- | ||
| + | |TERT | ||
| + | | | ||
| + | |Telomerase | ||
| + | | | ||
| + | |25% <ref name=":5" /> | ||
| + | |- | ||
| + | |EGFR | ||
| + | | | ||
| + | |Oncogene | ||
| + | |Amplification | ||
| + | |6% <ref name=":5" /> | ||
|} | |} | ||
| Line 114: | Line 136: | ||
==Epigenomics (Methylation)== | ==Epigenomics (Methylation)== | ||
| − | + | * MGMT promoter methylation may help predict response to alkylating chemotherapy <ref>{{Cite journal|last=Hegi|first=Monika E.|last2=Diserens|first2=Annie-Claire|last3=Gorlia|first3=Thierry|last4=Hamou|first4=Marie-France|last5=de Tribolet|first5=Nicolas|last6=Weller|first6=Michael|last7=Kros|first7=Johan M.|last8=Hainfellner|first8=Johannes A.|last9=Mason|first9=Warren|date=2005|title=MGMT gene silencing and benefit from temozolomide in glioblastoma|url=https://www.ncbi.nlm.nih.gov/pubmed/15758010|journal=The New England Journal of Medicine|volume=352|issue=10|pages=997–1003|doi=10.1056/NEJMoa043331|issn=1533-4406|pmid=15758010}}</ref> | |
==Genes and Main Pathways Involved== | ==Genes and Main Pathways Involved== | ||
| Line 122: | Line 144: | ||
==Diagnostic Testing Methods== | ==Diagnostic Testing Methods== | ||
| − | + | * Diagnosis typically made based on morphology with immunohistochemical staining | |
| + | ** GCG are typically distinguished from pleomorphic xanthastrocytoma by their lack of neuronal immunoreactivity <ref name=":7" /> | ||
| + | * Genomic testing for 1p/19q codeletion may help distinguish from oligodendroma | ||
==Clinical Significance (Diagnosis, Prognosis and Therapeutic Implications)== | ==Clinical Significance (Diagnosis, Prognosis and Therapeutic Implications)== | ||
| − | + | * GCG's have a poor prognosis, but may have better outcome than conventional IDH-wt GB <ref>{{Cite journal|last=Burger|first=P. C.|last2=Vollmer|first2=R. T.|date=1980|title=Histologic factors of prognostic significance in the glioblastoma multiforme|url=https://www.ncbi.nlm.nih.gov/pubmed/6260329|journal=Cancer|volume=46|issue=5|pages=1179–1186|doi=10.1002/1097-0142(19800901)46:53.0.co;2-0|issn=0008-543X|pmid=6260329}}</ref> <ref>{{Cite journal|last=Huang|first=M. C.|last2=Kubo|first2=O.|last3=Tajika|first3=Y.|last4=Takakura|first4=K.|date=1996|title=A clinico-immunohistochemical study of giant cell glioblastoma|url=https://www.ncbi.nlm.nih.gov/pubmed/8916121|journal=Noshuyo Byori = Brain Tumor Pathology|volume=13|issue=1|pages=11–16|issn=0914-8108|pmid=8916121}}</ref> <ref name=":2" /> <ref>{{Cite journal|last=Oh|first=Taemin|last2=Rutkowski|first2=Martin J.|last3=Safaee|first3=Michael|last4=Sun|first4=Matthew Z.|last5=Sayegh|first5=Eli T.|last6=Bloch|first6=Orin|last7=Tihan|first7=Tarik|last8=Parsa|first8=Andrew T.|date=2014|title=Survival outcomes of giant cell glioblastoma: institutional experience in the management of 20 patients|url=https://www.ncbi.nlm.nih.gov/pubmed/25037316|journal=Journal of Clinical Neuroscience: Official Journal of the Neurosurgical Society of Australasia|volume=21|issue=12|pages=2129–2134|doi=10.1016/j.jocn.2014.04.011|issn=1532-2653|pmid=25037316}}</ref> <ref name=":1" /> | |
| + | * Median survival 13.5 months vs 9.8 for conventional IDH-wt GB <ref name=":1" /> | ||
| + | * May be better circumscribed than conventional IDH-wt GB, making these lesions risky for radiographic misdiagnosis and delayed therapy <ref name=":6" /> | ||
==Familial Forms== | ==Familial Forms== | ||
| Line 139: | Line 165: | ||
Put your links here | Put your links here | ||
| + | |||
| + | ==Notes== | ||
==References== | ==References== | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
<nowiki>*</nowiki>Primary authors will typically be those that initially create and complete the content of a page. If a subsequent user modifies the content and feels the effort put forth is of high enough significance to warrant listing in the authorship section, please contact the CCGA coordinators (contact information provided on the homepage). Additional global feedback or concerns are also welcome. | <nowiki>*</nowiki>Primary authors will typically be those that initially create and complete the content of a page. If a subsequent user modifies the content and feels the effort put forth is of high enough significance to warrant listing in the authorship section, please contact the CCGA coordinators (contact information provided on the homepage). Additional global feedback or concerns are also welcome. | ||
| + | <references /> | ||
Latest revision as of 11:58, 13 April 2020
Primary Author(s)*
Jay Alden, DO
Cancer Category/Type
- Diffuse astrocytic and olidodendroglial tumors
Cancer Sub-Classification / Subtype
Glioblastoma, IDH-wildtype (IDH-wt)
Definition / Description of Disease
- Rare histologic variant of IDH-wt glioblastoma [1]
- Large, multinucleate giant cells with occasional abundant reticuln network [1]
Synonyms / Terminology
Epidemiology / Prevalence
- Constitute <1% of glioblastomas [2]
- May be more common in pediatric population [3]
- Mean age 51 years [3]
Clinical Features
- No evidence of a precursor lesion [1]
- Presenting symptoms similar to IDH-wt glioblastoma
- Radiographically and grossly circumscribed borders, may be mistaken for other neoplastic or non-neoplastic lesions [4]
Sites of Involvement
Morphologic Features
- Bizarre, multi-nucleate giant cells with atypical mitotic figures [5]
- Cells may contain numerous nuclei [1]
- Palisading and ischemic necrosis [1]
- Pseudo-rosette like perivascular tumor cell concentration [1]
Immunophenotype
| Finding | Marker |
|---|---|
| Positive (by definition) | IDH-1 |
| Positive (variable level) | GFAP [6] |
| Positive (Majority) | P53 [7] [6] |
| Negative (nearly universal) | Neuronal Antigens [8] |
Chromosomal Rearrangements (Gene Fusions)
Put your text here and/or fill in the table
| Chromosomal Rearrangement | Genes in Fusion (5’ or 3’ Segments) | Pathogenic Derivative | Prevalence |
|---|---|---|---|
| EXAMPLE t(9;22)(q34;q11.2) | EXAMPLE 3'ABL1 / 5'BCR | EXAMPLE der(22) | EXAMPLE 5% |
| EXAMPLE t(8;21)(q22;q22) | EXAMPLE 5'RUNX1 / 3'RUNXT1 | EXAMPLE der(8) | EXAMPLE 5% |
Characteristic Chromosomal Aberrations / Patterns
Put your text here
Genomic Gain/Loss/LOH
- Near haploidization may be a primary oncogenic event underlying a subset of GCG's [9]
- Recurrent loss of chromosomes 1, 2, 3, 4, 5, 6, 8, 9, 10, 11, 13, 14, 15, 17, 18, 19, 22
- Typically have gains or retain heterozygosity of chromosome 7
- Similar genomic findings have been described in other oncocytic tumors [10] [11] [12] [13]
- Oncogenenesis in such cases may be related to widespread loss of tumor suppressors in a catastrophic reduplication event early in tumorigenesis [10]
Gene Mutations (SNV/INDEL)
Put your text here and/or fill in the tables
| Gene | Mutation | Oncogene/Tumor Suppressor/Other | Presumed Mechanism (LOF/GOF/Other; Driver/Passenger) | Prevalence (COSMIC/TCGA/Other) |
|---|---|---|---|---|
| TP53 | Tumor Suppressor | 75-90% [14] | ||
| PTEN | Tumor Suppressor | 33% [15] | ||
| ATRX | Tumor Suppressor | Expression loss | 19% [15] | |
| TERT | Telomerase | 25% [15] | ||
| EGFR | Oncogene | Amplification | 6% [15] |
Other Mutations
| Type | Gene/Region/Other |
|---|---|
| Concomitant Mutations | EXAMPLE IDH1 R123H |
| Secondary Mutations | EXAMPLE Trisomy 7 |
| Mutually Exclusive | EXAMPLE EGFR Amplification |
Epigenomics (Methylation)
- MGMT promoter methylation may help predict response to alkylating chemotherapy [16]
Genes and Main Pathways Involved
Put your text here
Diagnostic Testing Methods
- Diagnosis typically made based on morphology with immunohistochemical staining
- GCG are typically distinguished from pleomorphic xanthastrocytoma by their lack of neuronal immunoreactivity [8]
- Genomic testing for 1p/19q codeletion may help distinguish from oligodendroma
Clinical Significance (Diagnosis, Prognosis and Therapeutic Implications)
- GCG's have a poor prognosis, but may have better outcome than conventional IDH-wt GB [17] [18] [3] [19] [2]
- Median survival 13.5 months vs 9.8 for conventional IDH-wt GB [2]
- May be better circumscribed than conventional IDH-wt GB, making these lesions risky for radiographic misdiagnosis and delayed therapy [4]
Familial Forms
Put your text here
Other Information
Put your text here
Links
Put your links here
Notes
References
*Primary authors will typically be those that initially create and complete the content of a page. If a subsequent user modifies the content and feels the effort put forth is of high enough significance to warrant listing in the authorship section, please contact the CCGA coordinators (contact information provided on the homepage). Additional global feedback or concerns are also welcome.
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 WHO Classification of Tumours of the Central Nervous System, 4th ed, Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (Eds), IARC, Lyon 2016. Pg 46-47
- ↑ 2.0 2.1 2.2 2.3 Ortega, Alicia; et al. (2014). "Treatment and survival of patients harboring histological variants of glioblastoma". Journal of Clinical Neuroscience: Official Journal of the Neurosurgical Society of Australasia. 21 (10): 1709–1713. doi:10.1016/j.jocn.2014.05.003. ISSN 1532-2653. PMID 24980627.
- ↑ 3.0 3.1 3.2 3.3 Kozak, Kevin R.; et al. (2009). "Giant cell glioblastoma: a glioblastoma subtype with distinct epidemiology and superior prognosis". Neuro-Oncology. 11 (6): 833–841. doi:10.1215/15228517-2008-123. ISSN 1523-5866. PMC 2802403. PMID 19332771.
- ↑ 4.0 4.1 Turner, Ryan; et al. (2018). "Imaging findings in the progression of a giant cell glioblastoma". Radiology Case Reports. 13 (5): 1007–1011. doi:10.1016/j.radcr.2018.07.010. ISSN 1930-0433. PMC 6097408. PMID 30128062.
- ↑ Margetts, J. C.; et al. (1989). "Giant-celled glioblastoma of brain. A clinico-pathological and radiological study of ten cases (including immunohistochemistry and ultrastructure)". Cancer. 63 (3): 524–531. doi:10.1002/1097-0142(19890201)63:33.0.co;2-d. ISSN 0008-543X. PMID 2912529.
- ↑ 6.0 6.1 Katoh, M.; et al. (1995). "Immunohistochemical analysis of giant cell glioblastoma". Pathology International. 45 (4): 275–282. doi:10.1111/j.1440-1827.1995.tb03456.x. ISSN 1320-5463. PMID 7550996.
- ↑ Peraud, A.; et al. (1997). "p53 mutations versus EGF receptor expression in giant cell glioblastomas". Journal of Neuropathology and Experimental Neurology. 56 (11): 1236–1241. doi:10.1097/00005072-199711000-00008. ISSN 0022-3069. PMID 9370234.
- ↑ 8.0 8.1 Martinez-Diaz, Hilda; et al. (2003). "Giant cell glioblastoma and pleomorphic xanthoastrocytoma show different immunohistochemical profiles for neuronal antigens and p53 but share reactivity for class III beta-tubulin". Archives of Pathology & Laboratory Medicine. 127 (9): 1187–1191. doi:10.1043/1543-2165(2003)1272.0.CO;2. ISSN 1543-2165. PMID 12946225.
- ↑ Bigner, S. H.; et al. (1985). "A serially transplantable human giant cell glioblastoma that maintains a near-haploid stem line". Cancer Genetics and Cytogenetics. 18 (2): 141–153. doi:10.1016/0165-4608(85)90064-0. ISSN 0165-4608. PMID 3840409.
- ↑ 10.0 10.1 Ganly, Ian; et al. (2018). "Integrated Genomic Analysis of Hürthle Cell Cancer Reveals Oncogenic Drivers, Recurrent Mitochondrial Mutations, and Unique Chromosomal Landscapes". Cancer Cell. 34 (2): 256–270.e5. doi:10.1016/j.ccell.2018.07.002. ISSN 1878-3686. PMC 6247912. PMID 30107176.
- ↑ Corver, Willem E.; et al. (2012). "Genome haploidisation with chromosome 7 retention in oncocytic follicular thyroid carcinoma". PloS One. 7 (6): e38287. doi:10.1371/journal.pone.0038287. ISSN 1932-6203. PMC 3365880. PMID 22675538.
- ↑ Corver, Willem E.; et al. (2014). "Near-haploidization significantly associates with oncocytic adrenocortical, thyroid, and parathyroid tumors but not with mitochondrial DNA mutations". Genes, Chromosomes & Cancer. 53 (10): 833–844. doi:10.1002/gcc.22194. ISSN 1098-2264. PMID 24909752.
- ↑ Gopal, Raj K.; et al. (2018). "Widespread Chromosomal Losses and Mitochondrial DNA Alterations as Genetic Drivers in Hürthle Cell Carcinoma". Cancer Cell. 34 (2): 242–255.e5. doi:10.1016/j.ccell.2018.06.013. ISSN 1878-3686. PMC 6121811. PMID 30107175.
- ↑ Martinez, Ramon; et al. (2007). "Cytogenetic and molecular genetic analyses of giant cell glioblastoma multiforme reveal distinct profiles in giant cell and non-giant cell subpopulations". Cancer Genetics and Cytogenetics. 175 (1): 26–34. doi:10.1016/j.cancergencyto.2007.01.006. ISSN 0165-4608. PMID 17498554.
- ↑ 15.0 15.1 15.2 15.3 Oh, Ji Eun; et al. (2016). "Genetic Alterations in Gliosarcoma and Giant Cell Glioblastoma". Brain Pathology (Zurich, Switzerland). 26 (4): 517–522. doi:10.1111/bpa.12328. ISSN 1750-3639. PMID 26443480.
- ↑ Hegi, Monika E.; et al. (2005). "MGMT gene silencing and benefit from temozolomide in glioblastoma". The New England Journal of Medicine. 352 (10): 997–1003. doi:10.1056/NEJMoa043331. ISSN 1533-4406. PMID 15758010.
- ↑ Burger, P. C.; et al. (1980). "Histologic factors of prognostic significance in the glioblastoma multiforme". Cancer. 46 (5): 1179–1186. doi:10.1002/1097-0142(19800901)46:53.0.co;2-0. ISSN 0008-543X. PMID 6260329.
- ↑ Huang, M. C.; et al. (1996). "A clinico-immunohistochemical study of giant cell glioblastoma". Noshuyo Byori = Brain Tumor Pathology. 13 (1): 11–16. ISSN 0914-8108. PMID 8916121.
- ↑ Oh, Taemin; et al. (2014). "Survival outcomes of giant cell glioblastoma: institutional experience in the management of 20 patients". Journal of Clinical Neuroscience: Official Journal of the Neurosurgical Society of Australasia. 21 (12): 2129–2134. doi:10.1016/j.jocn.2014.04.011. ISSN 1532-2653. PMID 25037316.