Difference between revisions of "MDS/AML with CUX1 deletion or del(7q22)"
| [unchecked revision] | [unchecked revision] |
| Line 50: | Line 50: | ||
o NGS for ''CUX1'' mutations | o NGS for ''CUX1'' mutations | ||
| − | [[File: | + | [[File:CUX1_deletion.jpg|center]] |
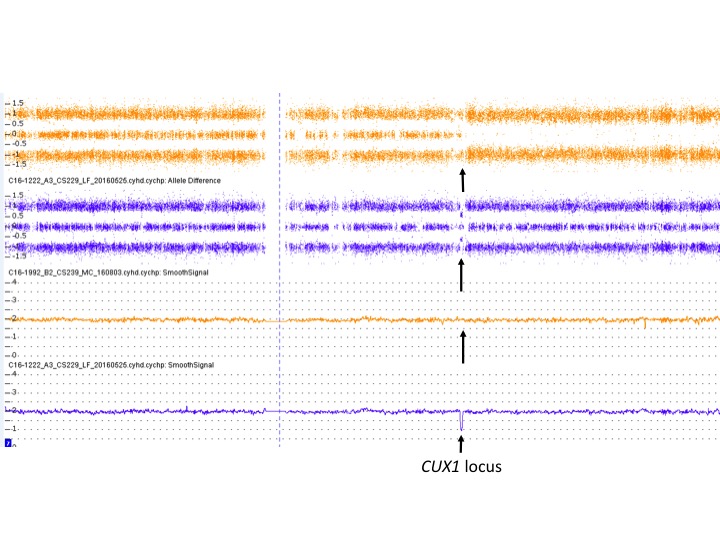
'''Figure 1.''' Chromosome genomic array testing (CGAT) demonstrating a small deletion of 7q and a large copy-neutral loss of heterozygosity of 7q. Bone marrow samples were analyzed using CytoScan HD (Affymetrix Inc.). The allelic tracks of chromosome 7 are shown in the upper panels and the smoothed copy number tracks are shown in lower panels. The arrows point to the ''CUX1'' gene locus at 7q22.1. One patient (in blue color, with the diagnosis of chronic myelomonocytic leukemia) displayed a 439-Kb deletion of ''CUX1'' as the sole abnormality. Another patient (in orange color, with the diagnosis of MDS) showed a terminal 7q cnLOH starting at the ''CUX1'' locus, also as the sole abnormality. (Image provided by M. Fang; copyright Fred Hutchinson Cancer Research Center.) | '''Figure 1.''' Chromosome genomic array testing (CGAT) demonstrating a small deletion of 7q and a large copy-neutral loss of heterozygosity of 7q. Bone marrow samples were analyzed using CytoScan HD (Affymetrix Inc.). The allelic tracks of chromosome 7 are shown in the upper panels and the smoothed copy number tracks are shown in lower panels. The arrows point to the ''CUX1'' gene locus at 7q22.1. One patient (in blue color, with the diagnosis of chronic myelomonocytic leukemia) displayed a 439-Kb deletion of ''CUX1'' as the sole abnormality. Another patient (in orange color, with the diagnosis of MDS) showed a terminal 7q cnLOH starting at the ''CUX1'' locus, also as the sole abnormality. (Image provided by M. Fang; copyright Fred Hutchinson Cancer Research Center.) | ||
Revision as of 21:18, 6 August 2016
Contributors
Rashmi Kanagal-Shamanna, MD, M.D. Anderson Cancer Center and Min Fang, MD, PhD, Fred Hutchinson Cancer Research Center
Description
Deletions of 7q are observed in a variety of myeloid neoplasms including de novo and secondary MDS/AML including therapy related forms. Monosomy 7 (-7) or del(7q) is regarded as an adverse prognostic marker with a median survival of 6 months [1].
Deletions of chromosome 7/ 7q are usually large spanning numerous genes. Three commonly deleted regions (CDRs) have been identified in myeloid neoplasms with 7q deletions: 7q22 (CDR1), 7q34 (CDR2) and 7q35-7q36.1 (CDR3) [2, 3]. For CDR1 region, multiple studies on AML transformed from MPN have identified the minimal deleted region to include CUX1 gene [4-6]. CUX1, also known as (CUT-like homeobox 1), is a gene that encodes transcription factor presumed to be the regulator for genes involved in cell cycle control and is a target for loss of heterozygosity (LOH) on chromosome 7q22.1 [2, 7]. McNerney et al. confirmed the role of CUX1 as a haploinsufficient tumor suppressor gene in de novo and therapy related myeloid neoplasms with del(7q) using a combination of SNP arrays and transcriptome analysis. The authors were able to demonstrate the reduction in CUX1 expression at half the usual levels and confirm the role of CUX1 in tumor development in vivo [2]. In hematopoietic neoplasms, CUX1 deletions are far more frequent than gains (8.3% loss versus 0.5% gain) [8]. However, in solid tumors, amplifications and gains of CUX1 associated with increased expression has been noted in cancers of CNS, endometrium, kidney, lung and ovaries suggesting its role as an oncogene [8].
Novel technologies using SNP-containing genomic arrays and next generation sequencing (NGS) allow the identification of very small del(7q) cases, copy neutral loss of heterozygosity of 7q (7q cnLOH), and mutations of CUX1. Patients with these abnormalities previously showed normal cytogenetics and were considered to have intermediate risk. Increasing evidence now demonstrate that these patients are of poor prognosis, similar to patients with -7/del(7q).
Tumor Types
Deletion of CUX1 is observed in AML and MDS (therapy-related and de novo), MDS/MPN, MPN, secondary AML transformed from Philadelphia chromosome-negative myeloproliferative neoplasms (MPNs), uterine leiomyomas, and breast cancer [2, 7-9]. CUX1 mutation is noted in endometrial, colonic (5%) and lung adenocarcinoma [8].
Prevalence
CUX1 deletion and mutations are observed at variable frequencies in different entities [7, 9, 10]. Focusing on myeloid neoplasms, deletions and mutations occur in ~11% overall cases:
- De novo MDS (5-10%), more prevalent in high-risk MDS than in low-risk ;
- AML (15-25%), more prevalent in secondary AML than in de novo AML;
- Therapy-related myeloid neoplasms (40-50%);
- MDS/MPN (13%)
- MPN (6%)
Concomitant Gene Mutations Involved / Domains Affected
Mutations of CUX1 do not appear to aggregate in any of its 33 exons or introns. In cases with monoallelic deletion of 7q22 (CUX1), mutation of the remaining CUX1 allele is rare [2, 11]. Occasionally, homozygous deletion and heterozygous mutations have been reported in AML transformed from MPN and CMML respectively [6, 12]. Whole exome sequencing studies on myeloid neoplasms with -7/del(7q)/7q cnLOH demonstrated that 7q cnLOH had more frequent somatic mutations than cases with -7/del(7q). About 17% of these mutations were noted in the CDR region genes: CUX1 (7q22), LUC7L2 (7q34), and EZH2 (7q35-36); and they were not mutually exclusive [9]. These concomitant mutations are analogous to the large size -7/del(7q) cases frequently seen encompassing all three CDRs. Frequent mutations noted in MDS/AML with CUX1 deletion/7q cnLOH include TET2, U2AF1/ U2AF2 (spliceosome genes), and TP53; but TP53 mutation was not identified in patients with concomitantly mutated CUX1, LUC7L2, and EZH2 [9].
Diagnostic Testing
o Metaphase cytogenetics (Karyotype) for 7q deletion greater than 7 Mb in size
o FISH for 7q22 deletion of 200 Kb or larger in size
o Chromosome genomic array testing (CGAT) with SNP/CN arrays to identify CUX1 deletion of 50-100 Kb or larger and to identify 7q cnLOH (Figure 1)
o NGS for CUX1 mutations
Figure 1. Chromosome genomic array testing (CGAT) demonstrating a small deletion of 7q and a large copy-neutral loss of heterozygosity of 7q. Bone marrow samples were analyzed using CytoScan HD (Affymetrix Inc.). The allelic tracks of chromosome 7 are shown in the upper panels and the smoothed copy number tracks are shown in lower panels. The arrows point to the CUX1 gene locus at 7q22.1. One patient (in blue color, with the diagnosis of chronic myelomonocytic leukemia) displayed a 439-Kb deletion of CUX1 as the sole abnormality. Another patient (in orange color, with the diagnosis of MDS) showed a terminal 7q cnLOH starting at the CUX1 locus, also as the sole abnormality. (Image provided by M. Fang; copyright Fred Hutchinson Cancer Research Center.)
Clinical Significance
Although -7/del(7q), when detected by karyotype and FISH, is well accepted as a poor prognostic marker in MDS/AML, smaller 7q deletions and cnLOH identified by CGAT are less recognized for their prognostic significance. With a 428-patient sample set, Hosono et al. demonstrated that CUX1 mutations/deletions are associated with a shorter overall survival (HR=2.25) [9]. The outcome is worse in patients with deletions compared to mutations [8, 12]. There is no specific association with other clinical or laboratory characteristics [9].
Therapeutics
Aggressive treatment strategies are appropriate for MDS/AML patients with CUX1 abnormalities, similar to those with -7/del(7q). High intensity chemotherapy, clinical trial with novel agents, and hematopoietic cell transplantation in first remission may be considered.
Links
References
1. Byrd, J.C., et al., Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood, 2002. 100(13): p. 4325-36.
2. McNerney, M.E., et al., CUX1 is a haploinsufficient tumor suppressor gene on chromosome 7 frequently inactivated in acute myeloid leukemia. Blood, 2013. 121(6): p. 975-83.
3. Jerez, A., et al., Loss of heterozygosity in 7q myeloid disorders: clinical associations and genomic pathogenesis. Blood, 2012. 119(25): p. 6109-17.
4. Klampfl, T., et al., Genome integrity of myeloproliferative neoplasms in chronic phase and during disease progression. Blood, 2011. 118(1): p. 167-76.
5. Thoennissen, N.H., et al., Prevalence and prognostic impact of allelic imbalances associated with leukemic transformation of Philadelphia chromosome-negative myeloproliferative neoplasms. Blood, 2010. 115(14): p. 2882-90.
6. Thoennissen, N.H., et al., Novel CUX1 missense mutation in association with 7q- at leukemic transformation of MPN. Am J Hematol, 2011. 86(8): p. 703-5.
7. Pellagatti, A. and J. Boultwood, The molecular pathogenesis of the myelodysplastic syndromes. Eur J Haematol, 2015. 95(1): p. 3-15.
8. Ramdzan, Z.M. and A. Nepveu, CUX1, a haploinsufficient tumour suppressor gene overexpressed in advanced cancers. Nat Rev Cancer, 2014. 14(10): p. 673-82.
9. Hosono, N., et al., Recurrent genetic defects on chromosome 7q in myeloid neoplasms. Leukemia, 2014. 28(6): p. 1348-51.
10. Papaemmanuil, E., et al., Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood, 2013. 122(22): p. 3616-27; quiz 3699.
11. Hindersin, S., et al., Mutation analysis of CUTL1 in childhood myeloid neoplasias with monosomy 7. Leuk Res, 2007. 31(9): p. 1323-4.
12. Wong, C.C., et al., Inactivating CUX1 mutations promote tumorigenesis. Nat Genet, 2014. 46(1): p. 33-8.